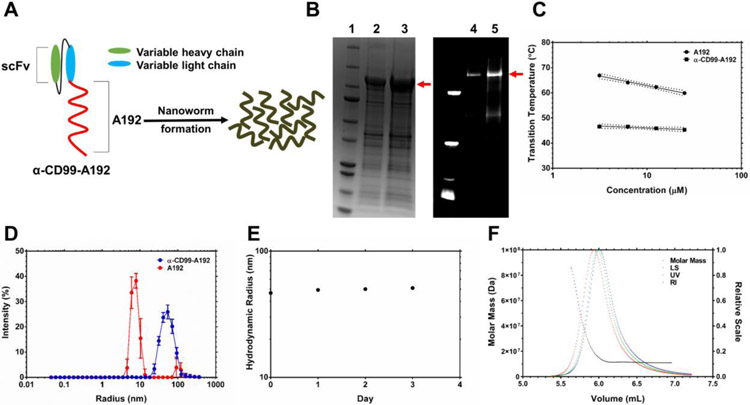
Figure 1. Construction, purification and refolding of a recombinant elastin-like polypeptide (ELP) fusion protein targeting CD99 receptors.
A) To construct an ELP fusion protein targeting CD99 receptors, α-CD99 scFv was fused to the N-terminus of a high molecular weight ELP, A192. It has been frequently observed that scFv-ELP fusion proteins form nanoworms spontaneously in PBS. B) The ELP fusion protein was purified by using ELP-mediated temperature-dependent phase separation. Three rounds of hot and cold temperature were used to purify α-CD99-A192, and the purity of the fusion protein was calculated to be 73.5 % based on the analysis of the SDS-PAGE gel on the left side: lane 1: ladder lane 2: 10 μg of α-CD99-A192 lane 3: 20 μg of α-CD99-A192. The SDS-PAGE gel on the right side shows the purity of NHS-rhodamine labeled α-CD99-A192, and the purity was calculated to be 77 %: lane 4: 8.5 μg of NHS-rhodamine labeled α-CD99-A192 lane 5: 17 μg of NHS-rhodamine labeled α-CD99-A192 C) The transition temperature of A192 and α-CD99-A192 was measured at 3.125, 6.25, 12.5, and 25 μM. The fusion of α-CD99 scFv to A192 substantially lowers the transition temperature of A192, and the transition temperature is less affected by the change of the protein’s concentration. Transition temperature becomes less sensitive to the change of the protein’s concentration. D) Hydrodynamic radius (Rh) of refolded α-CD99-A192 was measured with DLS at 37 °C. While A192 is monomeric, α-CD99-A192 formed nanoworms. E) The stability of α-CD99-A192 in PBS was observed with DLS for 72 hours. The Rh of the fusion protein did not change much over 72 hours, indicating that the fusion protein remains stable colloids in PBS at 37 °C. F) To measure the absolute molar mass of α-CD99-A192 nanoworms, SEC-MALS was employed. The average molecular weight of a nanoworm was measured to be 1.7×107 Da, which is equivalent to the molecular weight of 171 α-CD99-A192 molecules.