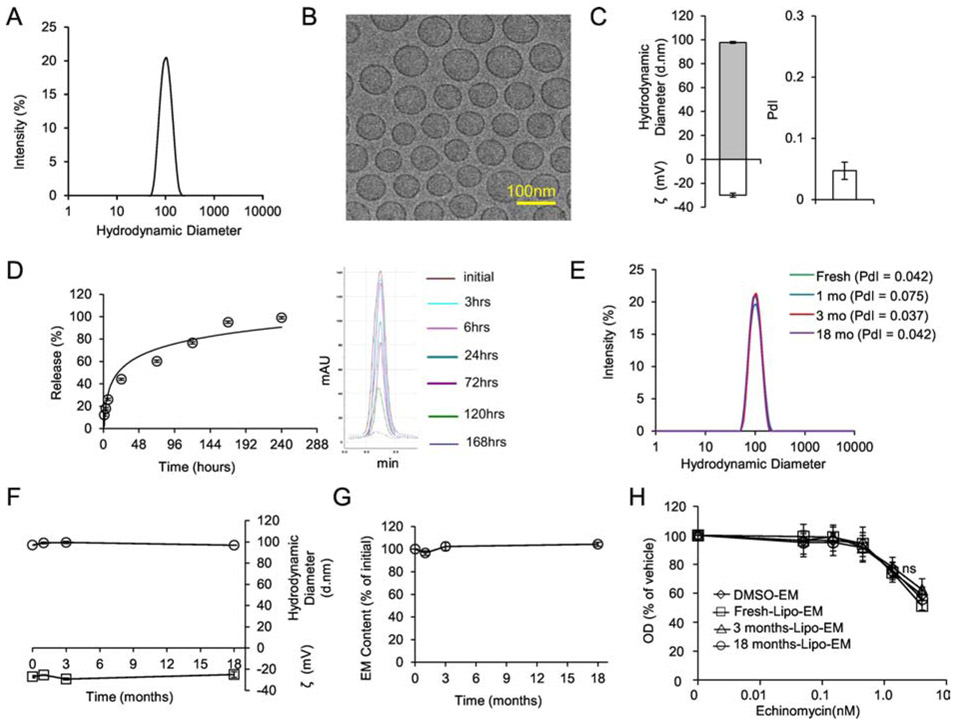
Figure 2.
Formulation and Characterization of Liposomal-echinomycin.
A) Size distribution of liposomal-echinomycin measured by DLS. B) Representative TEM depicting liposomal-echinomycin. C) Hydrodynamic diameter, zeta potential (ζ), and polydispersity index (PdI) of liposomal-echinomycin. Data shown as mean ± SD for 6 batches (detailed in Table S2). D) Release echinomycin from liposomal-echinomycin by dialysis. Percent release over time summarized as mean ± SD for triplicate runs on HPLC (Left), corresponding HPLC chromatograms are depicted (Right). E – H) Storage/Stability of liposomal-echinomycin. Liposomal-echinomycin was stored for 18 months at 4°C and analyzed at various time points for DLS size distribution (E), PdI (D, annotated), hydrodynamic diameter and ζ (F), echinomycin content loss (G), and potency against SUM-159 cells in vitro (24 hour incubation, performed in triplicate wells), determined by MTT assay (H). Data for D-G shown as mean ± SEM. EM, echinomycin.