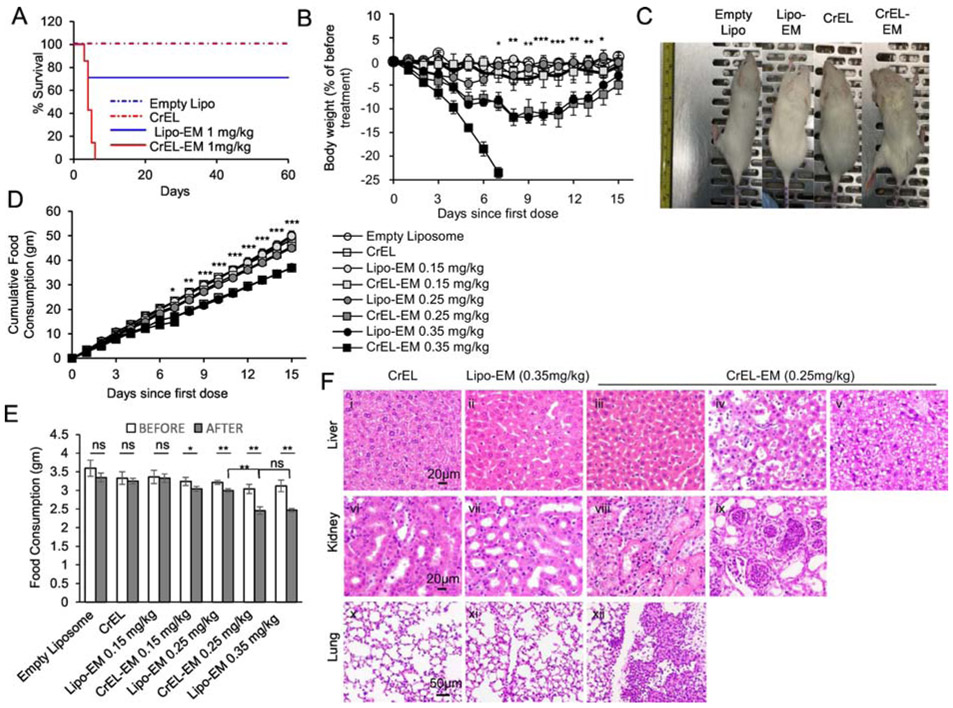
Figure 3.
Liposomal-echinomycin Is Less Toxic than CrEL-Echinomycin.
A) Survival of mice receiving single dose of CrEL-echinomycin or liposomal-echinomycin at 1 mgkg−1, or equivalent excipient dose (n=7 mice/group), administered day 0. B – F) Acute toxicity of mice receiving CrEL-echinomycin or liposomal-echinomycin at 0.15 mgkg−, 0.25 mgkg−1 or 0.35 mgkg−1, or corresponding vehicle controls (n=5 mice/group). Agents were administered days 0, 3, and 6. B) Body weight loss of mice throughout therapeutic cycle. P-values shown for CrEL-echinomycin 0.25 mgkg−1 vs liposomal-echinomycin 0.25 mgkg−1. C) Representative photograph of mice taken on day 7. D) Longitudinal analysis of cumulative food consumption throughout therapeutic cycle. P-values indicate CrEL-echinomycin 0.25 mgkg−1 vs liposomal-echinomycin 0.25 mgkg−1. Comparison of average daily food consumption 3 days prior to (before) and 15 days following (after) dose one, shown as mean ± SEM. F) Representative H&E staining of liver, kidney, and lung tissues for mice on day 7. Panel i: CrEL-vehicle mouse liver showing normal glycogen levels; panel ii: liposomal-echinomycin 0.35 mgkg−1 mouse liver showing mild glycogen depletion; panel iii: p CrEL-echinomycin 0.25 mgkg−1 mouse liver showing glycogen depletion with mild atrophy, reduced hepatocyte size; panels iv, v: CrEL-echinomycin 0.25 mgkg−1 mice livers showing hepatitis (panel iv) and steatosis (panel v); panel vi: normal kidney, CrEL-vehicle mouse; panel vii: normal kidney, liposomal-echinomycin 0.35 mgkg−1 mouse; panels viii, ix: abnormal kidneys from CrEL-echinomycin mice showing massive necrosis of proximal tubule epithelium (panel viii) and pyelonephritis (panel ix); panel x: normal lung, CrEL-vehicle mouse; panel xi: normal lung, liposomal-echinomycin 0.35 mgkg−1 mouse; panel xii: abnormal lung from CrEL-echinomycin 0.25 mgkg−1 mouse showing multi-focal pleuritis with mineralization, hemorrhage, and necrosis.