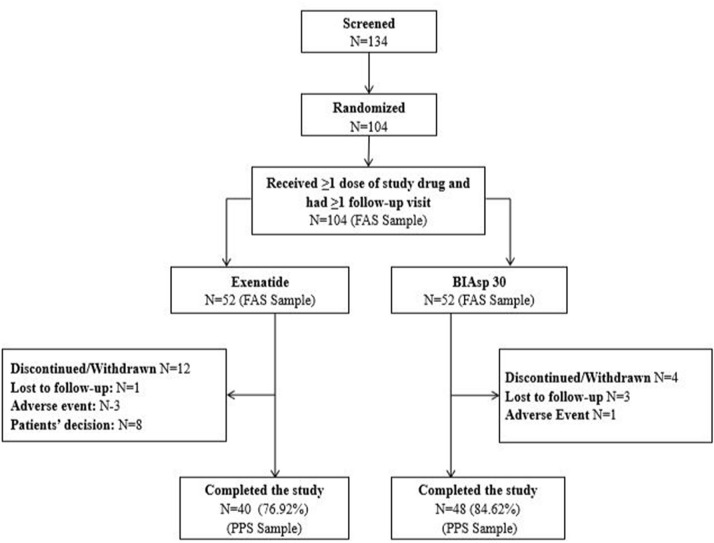
Fig. 1.
Flow of participants through the study. FAS full analysis set included all randomized participants receiving at least one dose of any of the trial products. PAS per protocol set, the data set was determined during a blind audit after the completion of the trial, including all cases that met the trial protocol, had good compliance, had not taken the prohibited drugs during the trial, and had completed the CRF requirements. PPS per protocol set, the population was the secondary population for efficacy evaluation in this study