Abstract
Drug therapies for people with heart failure and preserved ejection fraction (HFpEF) are often limited to diuretics to improve symptoms as no therapies demonstrate a mortality benefit in this cohort. People with diabetes have a high risk of developing HFpEF and vice versa, suggesting shared pathophysiological mechanisms exist, which in turn engenders the potential for shared treatments. Dapagliflozin is a sodium–glucose co-transporter 2 (SGLT2) inhibitor which has demonstrated significantly improved cardiovascular and hospitalisation for heart failure (HHF) outcomes in previous cardiovascular outcome trials (CVOTs). These CVOTs include the DECLARE-TIMI and DAPA-HF studies which observed significant benefits for people with heart failure and specifically those with heart failure and reduced ejection fraction (HFrEF), respectively. The ongoing DELIVER study is evaluating the use of dapagliflozin specifically in people with HFpEF, which may have enormous implications for treatment and considerable economic consequences. This will complement previous and other ongoing CVOTs evaluating dapagliflozin use. In this review we discuss the use of SGLT2 inhibitors in HFrEF and HFpEF with a focus on the DELIVER study and its potential health and economic implications.
Keywords: Dapagliflozin, DELIVER study, Heart failure, Preserved ejection fraction, SGLT2 inhibitors, Type 2 diabetes
Key Summary Points
| Dapagliflozin improved cardiovascular and hospitalization for heart failure (HHF) outcomes in people with heart failure and reduced ejection fraction (HFrEF) in the DAPA-HF study. |
| However, it remains unknown whether drug therapy including dapagliflozin or any sodium–glucose co-transporter 2 (SGLT2) inhibitor improves cardiovascular or HHF outcomes in people with heart failure and preserved ejection fraction (HFpEF). |
| The DELIVER study aims to determine the impact of dapagliflozin on cardiovascular death, HHF or urgent heart failure visit in people with HFpEF. |
| Treatments which reduce the rate of HHF may have the greatest economic impact since hospitalization accounts for the majority of heart failure treatment costs. |
Introduction
Heart failure is an increasingly recognised diagnosis in people with diabetes and its prevalence is increasing, with an estimated 26 million people diagnosed worldwide and an estimated global economic burden of over US$108 billion [1, 2]. Whilst the economic impact of heart failure is substantial, the personal impact including recurrent hospitalisation and poorer quality of life outcomes compared with most chronic diseases is significant [1]. Moreover, the projected 5-year mortality associated with heart failure is high at around 75% [3]. People with heart failure can be broadly classified as those with reduced left ventricular ejection fraction (HFrEF) and those with preserved left ventricular ejection fraction (HFpEF) based on a left ventricular ejection fraction (LVEF) < 40% or > 40%, respectively. Whilst approximately 50% of people with heart failure have HFpEF, its relative prevalence is increasing and it is anticipated that HFpEF prevalence will soon exceed that of HFrEF [1, 4]. Crucially, whilst treatment with angiotensin converting enzyme (ACE) inhibitors, beta blockers or mineralocorticoid receptor antagonists, amongst others, reduces mortality in people with HFrEF, there is no known therapy which reduces mortality in people with HFpEF [5–7].
There is a strong association between heart failure and diabetes, and people with diabetes have a 2–5-fold greater lifetime risk of developing heart failure, whilst around 45% of people with heart failure have underlying diabetes [8, 9]. Indeed, heart failure is the most common first manifestation of cardiovascular disease in people with diabetes. The association with diabetes is greater with HFpEF than HFrEF, possibly a result of shared pathophysiological mechanisms including increased inflammation, aberrant angiogenesis and remodelling, impaired cardiac metabolism, altered insulin signalling and advanced glycated end-product deposition within the myocardium [10, 11]. Therefore, treatments which reduce or reverse these changes have a massive potential for impact in people with type 2 diabetes (T2D) and may improve cardiovascular outcomes in this high-risk population. Indeed, treatments which improve cardiovascular outcomes in people with T2D are highly sought and of topical interest with the undertaking of cardiovascular outcome trials (CVOTs) for all T2D drug therapies. Given that heart failure typically occurs in the elderly, the use of medications such as sodium–glucose co-transporter 2 (SGLT2) inhibitors to improve outcomes in this population is attractive. However, there is limited experience seen in clinical trials and real-world data to evaluate this approach. Here, we discuss the use of SGLT2 inhibitors for heart failure with a focus on dapagliflozin and the ongoing DELIVER trial evaluating dapagliflozin in people with HFpEF. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
SGLT2 Inhibitors Improve Cardiovascular and Renal Outcomes in CVOTs
Several SGLT2 inhibitors have been introduced for people with T2D, including canagliflozin, dapagliflozin, empagliflozin and ertugliflozin amongst others. These drugs inhibit the SGLT2 protein in the proximal convoluted tubule of the nephron to induce a glucose-mediated osmotic diuresis and natriuresis. As a result, these drugs are associated with important improvements in glycaemic control, blood pressure, body weight and lipids [12]. Additionally, CVOTs investigating the safety of these drugs have observed improved cardiovascular and heart failure outcomes associated with their use.
A recent meta-analysis of CVOTs reported a reduced rate of 3-point major adverse cardiac events (MACE) (hazard ratio (HR) 0.88, confidence interval (CI) 0.82–0.94), cardiovascular death (HR 0.83, CI 0.75–0.92) and all-cause mortality (HR 0.85, CI 0.79–0.92) associated with SGLT2 inhibitor use versus placebo [13]. Indeed, CVOTs have consistently observed important cardiovascular and/or heart failure benefits associated with SGLT2 inhibitor use [14–20]. However, studies have previously included variable participant groups with differing cardiovascular risk and utilise various definitions of HFpEF and HFrEF including LVEF and N-terminal pro B-type natriuretic peptide (NT-pro BNP) amongst other variables. This is crucial to consider when distinguishing between drugs in this class for people with heart failure. Trial participant characteristics and heart failure endpoints from ongoing and completed CVOTs reporting on heart failure outcomes are presented in Table 1.
Table 1.
Ongoing and completed placebo-controlled trials of SGLT2 inhibitors evaluating heart failure outcomes
| Drug/trial | Key inclusion criteria | Participants with HF at baseline | Definition of HF | HF outcomes |
|---|---|---|---|---|
|
Canagliflozin CANVAS programme [15] (n = 10,142) |
T2D [HbA1c 53–91 mmol/mol (7.0–10.5%)] Established CVD or high risk of CVD |
n = 1461 (14.4%) | Not defined | HHF: HR 0.67 (CI 0.52–0.87) |
|
Dapagliflozin |
T2D (HbA1c 6.5–12.0%) Established CVD or high risk of CVD |
HFrEF: 671 (3.9%) HFpEF: 1316 (7.7%) |
HFrEF: LVEF < 40% HFpEF: LVEF > 40% |
HHF: HR 0.73 (CI 0.61–0.88) HFrEF HR 0.64 (CI 0.43–0.95) HFpEF HR 0.76 (CI 0.62–0.94) |
|
Dapagliflozin DAPA-HF [18] (n = 4744) |
Symptomatic HFrEF |
n = 4744 (100%) |
LVEF < 40% NT-pro BNP > 600 pg/ml, or > 400 pg/ml if HHF < 1 year, or > 900 pg/ml if AF NYHA class II–IV |
HHF or CV death: HR 0.74 (CI 0.65–0.85) HHF: HR 0.70 (CI 0.59–0.83) Change in KCCQ: HR 1.18 (CI 1.11–1.26) |
|
Dapagliflozin DELIVER [33] (n = ~ 6100) |
Symptomatic HFpEF | n = ~ 6100 (100%) |
LVEF > 40% and structural heart disease Elevated NT-pro BNP |
Not yet reported |
|
Empagliflozin EMPA-REG [14] (n = 7020) |
T2D [HbA1c 53–86 mmol/mol (7.0–10.0%)] Established CVD |
n = 706 (10.1%) |
Not defined | HHF 0.65 (CI 0.50–0.85) |
|
Empagliflozin EMPEROR-reduced [31] (n = ~ 3730) |
Symptomatic HFrEF | n = ~ 3730 (100%) |
LVEF ≤ 40% Elevated NT-proBNP: EF 36–40%: ≥ 2500 pg/ml without AF; ≥ 5000 pg/ml with AF EF 31–35%: ≥ 1000 pg/ml without AF; ≥ 2000 pg/ml with AF EF ≤ 30%: ≥ 600 pg/ml without AF; ≥ 1200 pg/ml with AF EF ≤ 40% and HHF < 12 months: ≥ 600 pg/ml without AF; ≥ 1200 pg/ml with AF |
Not yet reported |
|
Empagliflozin EMPEROR-preserved [32] (n = ~ 5988) |
Symptomatic HFpEF | ~ 5988 participants (100%) |
LVEF > 40% and structural heart disease Elevated NT-proBNP >300 pg/ml without AF >900 pg/ml with AF |
Not yet reported |
|
Ertugliflozin |
T2D [HbA1c 53–91 mmol/mol (7.0–10.5%)] Established CVD |
Not yet reported | Not defined | HHF: 2.5% vs 3.6% in placebo group |
AF atrial fibrillation, CV cardiovascular, CVD cardiovascular disease, HbA1c glycated haemoglobin, HFrEF heart failure with reduced ejection fraction, HFpEF heart failure with preserved ejection fraction, HHF hospitalisation for heart failure, HR hazard ratio, KCCQ Kansas City Cardiomyopathy Questionnaire, LVEF left ventricular ejection fraction, NT-pro BNP N-terminal pro B-type natriuretic peptide, NYHA New York Heart Association, T2D type 2 diabetes
There has also been a great deal of interest in renal outcomes associated with SGLT2 inhibitors given their renal mechanism of action and the association of chronic kidney disease (CKD) in people with diabetes and cardiovascular disease. Diagnosis of CKD in people with heart failure is common, a result of chronic fluid overload and potential acute kidney injury associated with many treatments for heart failure causing the so-called cardio-renal syndrome [21]. Indeed, CVOTs investigating SGLT2 inhibitor use have reported that these drugs are associated with a delay in the decline in glomerular filtration rate (GFR) and a reduced frequency of progression to macroalbuminuria typically seen in people with enduring diabetes [22]. Given their nephroprotective impact, their use in heart failure is more appealing given the relatively high risk of developing cardio-renal syndrome and CKD.
The cardiovascular and renal benefits observed in these studies cannot be fully explained by improvements in risk factors such as glycaemic control, blood pressure or lipids [14], implying that other mechanisms must explain the cardiovascular benefits seen in HFrEF and possibly HFpEF. The most likely explanation is tubuloglomerular feedback. Here, SGLT2 inhibition results in the increased delivery of sodium (and glucose) to the macula densa, resulting in afferent arteriolar vasoconstriction to reduce the hyperfiltration which frequently characterises the earlier stages of diabetic nephropathy, thereby improving the CKD outcomes discussed above. This may explain how SGLT2 inhibitor-mediated diuresis improves heart failure outcomes also, whilst loop and thiazide diuretics do not improve cardiovascular outcomes. Indeed, loop and thiazide diuretics block sodium entry to the macula densa via the Na–Cl pump and thereby attenuate tubuloglomerular feedback [23, 24].
In addition to tubuloglomerular feedback, SGLT2 inhibitor use produces a greater fluid shift from the interstitial space resulting in improved congestion whilst not significantly affecting organ perfusion. Other authors speculate that SGLT2 inhibition results in a state of ‘fasting mimicry’ through enhancing glycosuria which can induce enzymes within the myocardium which have anti-inflammatory and antioxidant effects. Moreover, the augmented glycosuria results in an energy shift to enhanced ketone metabolism and inhibited cardiac sodium–hydrogen exchange. These effects improve myocardial energy metabolism which appears to reduce myocardial inflammation and fibrosis [25]. Whilst there are many possible mechanisms, the exact role of SGLT2 inhibition in ameliorating cardiovascular and heart failure outcomes is unclear. Figure 1 is a schematic summarising the most appealing of these mechanisms. Nevertheless, the relative influence of these mechanisms is debated as many are unsubstantiated in humans or argued to be an indirect effect of improved glycaemic control. Further investigation in this area is warranted to corroborate these potential mechanisms.
Fig. 1.
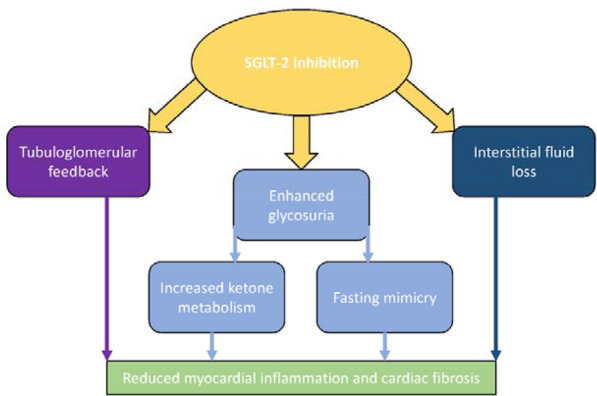
Key potential mechanisms by which SGLT2 inhibitors may improve heart failure and cardiovascular outcomes in people with HFrEF and HFpEF [23–25]. SGLT2 sodium–glucose co-transporter 2
Dapagliflozin for Heart Failure and Chronic Kidney Disease
The cardiovascular safety of dapagliflozin was first evaluated in the DECLARE-TIMI trial, in 17,160 people with T2D with pre-existing or high risk of developing cardiovascular disease over a median 4.2 years. Here, 10.0% of participants had a pre-existing heart failure diagnosis, though the proportion of those with HFrEF or HFpEF was not initially reported. Whilst dapagliflozin use did not meet superiority for 3-point MACE against placebo (HR 0.93, CI 0.84–1.03), it did reduce the risk of hospitalisation for heart failure (HHF) (HR 0.73, CI 0.61–0.88) [17]. A subsequent analysis of the DECLARE-TIMI trial outcomes by Kato et al. [26] observed that 3.9% of participants had HFrEF and 7.7% had HFpEF at baseline. Here, dapagliflozin reduced HHF in participants with HFrEF and HFpEF, and the effect was greater in those with HFrEF than HFpEF (HR 0.64 (CI 0.43–0.95) vs HR 0.76 (CI 0.62–0.94)). However, dapagliflozin use reduced cardiovascular and all-cause mortality in people with HFrEF, but not in those with HFpEF [26]. Moreover, dapagliflozin use was associated with a reduction in the number of participants observed to achieve the renal-specific outcome measure (HR 0.53, CI 0.43–0.66), including a sustained decline in GFR (HR 0.54, CI 0.43–0.67) and end-stage renal disease or renal death (HR 0.41, CI 0.20–0.82). Moreover, participants receiving dapagliflozin had a 29% lower mean urinary albumin-to-creatinine ratio (ACR) than those using placebo [27, 28]. Therefore, whilst dapagliflozin reduced the frequency of adverse heart failure and renal outcomes in this high-risk participant cohort, more specific evaluation in different cohorts was required to validate these findings further.
The subsequent DAPA-HF study investigated the use of dapagliflozin in 4744 people with HFrEF over a median 18.2 months and did not include participants with HFpEF. The study found a significant relative risk reduction compared with placebo for cardiovascular death (HR 0.82, CI 0.69-0.98) and HHF (HR 0.70, CI 0.59–0.83), with a similar impact in people with or without T2D [18]. Additionally, dapagliflozin use was associated with reduced frequency of developing the renal composite outcome (HR 0.71, CI 0.44-1.16) and renal adverse events (6.5% vs 7.2%) versus placebo. Whilst these results supported the recent Food and Drug Administration (FDA) approval for dapagliflozin use in people with HFrEF [29], as it did not include sufficient participants with HFpEF its efficacy in this patient group remains unknown and its use in this group is not approved.
The DAPA-CKD study is an ongoing trial to investigate the impact of dapagliflozin on GFR, changes in urinary ACR, cardiovascular death or HHF in around 4300 participants with CKD (baseline GFR 25–75 mL/min/1.73 m2) [30]. Whilst the baseline characteristics of the number of participants with underlying heart failure are currently unavailable, secondary outcome measures of the study include time to cardiovascular death and HHF. The trial was due to complete in June 2020, and we await the results of this trial with great interest.
The impact of dapagliflozin and SGLT2 inhibitors generally in people with HFpEF remains unknown and given the lack of outcome-changing therapies for people with HFpEF the potential impact in this patient group is enormous. The ongoing EMPEROR trials will further establish the impact of empagliflozin in people with HFrEF or HFpEF [31, 32], whilst the DELIVER trial will focus entirely on the cardiovascular and heart failure outcomes associated with dapagliflozin in participants with HFpEF [33].
The DELIVER Trial: What Will it Add?
The DELIVER trial is a phase III international, multicentre, parallel-group, randomised, double-blind, placebo-controlled study evaluating the impact of dapagliflozin 10 mg versus placebo in participants with HFpEF [33]. The study started in August 2018 and is due to complete in June 2021, with an estimated enrolment of 6100 participants. The primary outcome measure is the time to first occurrence of either cardiovascular death, HHF or urgent heart failure visit. Secondary outcome measures include the number of HHF and cardiovascular death, changes in the total symptom score measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ), the proportion of patients with worsened New York Heart Association (NYHA) class and time to the occurrence of death from any cause. Inclusion and exclusion criteria are presented in Table 2 and the study design is presented in Fig. 2.
Table 2.
Inclusion and exclusion criteria for the DELIVER study.
Table adapted from ClinicalTrials.gov [33]
| Inclusion | Exclusion |
|---|---|
| Aged ≥ 40 years |
Receiving an SGLT2 inhibitor < 4 weeks prior to randomisation Type 1 diabetes mellitus |
| Symptomatic HF (NYHA class II–IV) ≥ 6 weeks before enrolment and at least intermittent need for diuretics |
eGFR < 25 mL/min/1.73 m2 Systolic BP < 95 mmHg or ≥ 160 mmHg if not on ≥ 3 BP-lowering medications, or ≥ 180 mmHg irrespective of treatments |
| LVEF > 40% and evidence of structural heart disease documented by the recent echocardiogram and/or cardiac MR within the last 12 months | MI, unstable angina, coronary revascularization, AF ablation, valve repair/replacement < 12 weeks before enrolment |
| Elevated NT-pro BNP levels | Planned coronary revascularization, AF ablation or valve repair/replacement |
| Ambulatory and hospitalised patients can be enrolled. Patients hospitalised for HF must not receive intravenous HF medications for at least 24 h prior to enrolment |
Stroke or TIA < 12 weeks before enrolment Alternative diagnoses which could account for the patient’s HF symptoms and signs BMI > 50 kg/m2 |
BMI body mass index, BP blood pressure, eGFR estimated glomerular filtration rate, HF heart failure, LVEF left ventricular ejection fraction, MI myocardial infarction, NT-pro BNP N-terminal pro B-type natriuretic peptide, NYHA New York Heart Association, SGLT2 sodium–glucose co-transporter 2, TIA transient ischaemic attack
Fig. 2.
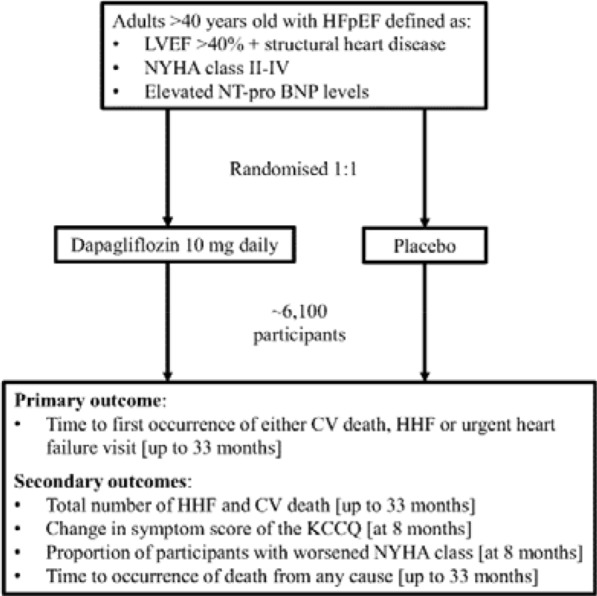
The trial design and key outcome measures from the DELIVER study. Figure adapted from ClinicalTrials.gov [33]. CV cardiovascular, HHF Hospitalisation for Heart Failure, KCCQ Kansas City Cardiomyopathy Questionnaire, LVEF left ventricular ejection fraction, NYHA New York Heart Association, NT-pro BNP N-terminal pro B-type natriuretic peptide
The DELIVER study runs in parallel with two other key trials evaluating dapagliflozin: The DAPA-HF study [18] and the DAPA-CKD study [30]. The findings from the DELIVER study will complement those of the DAPA-HF study and determine whether the impact of dapagliflozin on the rate of HHF and cardiovascular death in people with HFrEF is also reproduced in people with HFpEF. As discussed earlier, this would potentially have a major impact for people with HFpEF as no current drug therapy has yet demonstrated any cardiovascular outcome benefit in this patient group. The study is well powered for superiority with a large participant number expected to enrol. This adds significantly to the previous sub-analysis of 1316 participants with HFpEF in the DECLARE-TIMI trial, which may explain the limited findings and relative lack of study power in this relatively small cohort [26]. Moreover, as participants without underlying diabetes are included, data on incident diabetes in this group will be available thereby adding to the DAPA-HF study which reported a significant 32% relative risk reduction of incident diabetes in participants receiving dapagliflozin versus placebo [34]. Of course, should results favour dapagliflozin use the economic gains in addition to improved patient outcomes may be substantial given the increasing financial burden associated with HFpEF, discussed below.
Whilst we await the completion and publication of the results of the DAPA-CKD study [30], results from previous CVOTs evaluating dapagliflozin have shown the drug is both safe and probably improves renal outcomes in high-risk participants with cardiovascular disease [17, 18, 27, 28]. Thus, dapagliflozin would prove an attractive option for people with HFpEF who frequently develop renal complications and improve glycaemic control and incident diabetes in this population which frequently have or are at a very high risk of developing T2D.
The DELIVER and EMPEROR Studies: What Are the Differences?
As discussed briefly above, the EMPEROR studies are two distinct trials which aim to establish the effect of empagliflozin on heart failure and cardiovascular outcomes in people with HFrEF [31] and HFpEF [32], which we recently reviewed [35]. Given that these medications are from the same drug class, comparisons in trial results will naturally be made when available. The trials are broadly similar, aiming to establish the impact of an SGLT2 inhibitor in people with HFpEF in similar numbers of enrolled trial participants (5750–6100), as shown in Table 3. However, there are important differences between the trials also. For example, the DELIVER trial will include both ambulatory and hospitalised participants stable on oral therapy for at least 24 h. In contrast, the EMPEROR-preserved trial requires a greater duration of stability on oral therapy (> 1 week). This earlier initiation and in hospitalised participants may show changes in length of hospital stay which would have significant clinical and economic implications. However, the study may not be sufficiently powered to address this outcome. Moreover, the DELIVER trial includes participants with symptomatic HF for at least 6 weeks, compared with the EMPEROR-preserved trial in which participants are symptomatic for at least 3 months. Additionally, the DELIVER trial excludes people with BMI > 50 kg/m2, whilst EMPEROR-preserved excludes those with liver disease [32, 33].
Table 3.
Similarities and differences between the DELIVER and EMPEROR-preserved trials.
Table adapted from ClinicalTrials.gov [33] and Anker et al. [32]
| DELIVER trial (dapagliflozin) | EMPEROR-preserved (empagliflozin) | |
|---|---|---|
| Study participants | ~ 6100 | ~ 5988 |
| Anticipated follow-up and dates of the study | Up to ~ 33 months | Median ~ 24 months |
| August 2018–June 2021 | March 2017–November 2020 | |
| Inclusion criteria | Aged ≥ 40 years | Aged ≥ 18 years |
| Symptomatic HF (NYHA class II–IV) ≥ 6 weeks before enrolment and at least intermittent need for diuretics | Symptomatic HF (NYHA class II–IV) ≥ 3 months before enrolment on a stable dose of diuretics for 1 week, if prescribed | |
| Ambulatory and hospitalised patients can be enrolled, people with HHF cannot receive IV diuretics for ≥ 24 h prior to enrolment | Patients admitted with ADHF cannot be enrolled if ≤ 1 week of screening or screening period | |
| Definition of HFpEF | LVEF > 40% and structural heart disease documented by echocardiogram and/or cardiac MR ≤ 12 months | LVEF > 40% with structural heart disease ≤ 6 months, or HHF ≤ 12 months prior to visit 1 |
| Elevated NT-pro BNP levels |
Elevated NT-proBNP (a) > 300 pg/ml for patients without AF (b) > 900 pg/ml for patients with AF |
|
| Key differences in exclusion criteria | eGFR < 25 mL/min/1.73 m2 | eGFR < 20 ml/min/1.73 m2 or requiring dialysis |
| SBP < 95 mmHg or ≥ 160 mmHg if ≤ 3 BP drugs, or ≥ 180 mmHg irrespective of treatment | SBP ≥ 180 mmHg, symptomatic hypotension and/or SBP ≤ 100 mmHg | |
| People with ADHF can be enrolled if not received IV diuretics ≥ 24 h | ADHF requiring IV diuretics, vasodilator or mechanical support ≤ 1 week of screening or during the screening period | |
| MI, unstable angina, coronary revascularization, AF ablation, valve repair/replacement < 12 weeks | MI, CABG or major cardiovascular surgery, stroke or TIA in past 90 days prior to visit 1 | |
| Planned coronary revascularization, AF ablation or valve repair/replacement | ||
| Stroke or TIA < 12 weeks before enrolment | Heart transplant recipient, or listed for heart transplant | |
| BMI > 50 kg/m2 | Indication of liver disease | |
| Primary outcomes | Time to first occurrence of either cardiovascular death, HHF or urgent heart failure visit | Time to first occurrence of the combined risk for cardiovascular death or HHF |
| Secondary outcomes | Number of HHF and cardiovascular death | HHF events and cardiovascular death |
| Death from any cause | Death from any cause | |
| Proportion of patients with worsened NYHA class | All-cause hospitalisation | |
| Changes in clinical summary score of the KCCQ |
Changes in eGFR, time to chronic dialysis, renal transplant or sustained reduction of eGFR, time to onset of diabetes Changes in clinical summary score of the KCCQ |
ADHF acute decompensated heart failure, AF atrial fibrillation, CABG coronary artery bypass graft, eGFR estimated glomerular filtration rate, HHF hospitalisation for heart failure, IV intravenous, KCCQ Kansas City Cardiomyopathy Questionnaire, lvef left ventricular ejection fraction, MI myocardial infarction, MRI magnetic resonance imaging, NT-pro BNP N-terminal pro B-type natriuretic peptide, NYHA New York Heart Association, SBP systolic blood pressure, TIA transient ischaemic attack
The primary outcome in both studies includes the time to first occurrence of cardiovascular death or HHF, though the DELIVER trial also includes urgent heart failure visits such as to the emergency department or outpatient clinic in addition to hospitalisation. This is noteworthy, because whilst hospitalisation is a major determinant for quality of life and health expenditure outcomes, the use of extra outpatient or emergency department costs is often overlooked. As secondary outcomes, both trials will report on HHF, cardiovascular and all-cause death and quality of life outcomes using the Kansas City Cardiomyopathy Questionnaire (KCCQ). However, the EMPEROR-preserved trial will also report on changes in eGFR, time to chronic dialysis, renal transplant or sustained reduction of eGFR and rates of incident diabetes in those using empagliflozin whilst these secondary outcomes have not yet been pre-specified for the DELIVER trial [32, 33].
Cost-Effectiveness
The use of drugs which reduce HHF may have the greatest impact on healthcare expenditure for people with heart failure, as approximately 70% of the cost associated with the heart failure treatment results from hospitalisation, and around two-thirds of patients have at least one readmission within 1 year [36]. Whilst there is no published cost analysis undertaken by the DAPA-HF study group, several authors have analysed the potential cost saving associated with dapagliflozin use in people with HFrEF. An Australian study estimated the cost-effectiveness of dapagliflozin in people with HFrEF at $12,482 Australian dollars per quality-adjusted life year (QALY) versus the standard of care, with similar cost savings in people with or without T2D [37]. Furthermore, a Swedish study compared real-world healthcare costs associated with dapagliflozin use compared with people initiated on non-SGLT2 inhibitor glucose-lowering treatments. The study observed lower hospital costs of US$321 per patient over 12 months associated with dapagliflozin, mostly a result of fewer cardiovascular, heart failure and other T2D-related complications [38].
The impact of dapagliflozin or other SGLT2 inhibitors on HHF and other cardiovascular health outcomes in people with HFpEF is unknown, and therefore cost analyses are impossible. However, given that around 50% of people hospitalised for heart failure have underlying HFpEF [1, 39] and the cost of HHF is the largest contributor to heart failure treatment costs, any reduction in the HHF or length of hospital stay would incur major cost savings.
Conclusions
The use of dapagliflozin in the treatment of people with HFrEF will soon be commonplace following the results of the DAPA-HF study and recent FDA approval. The DELIVER study has the potential to deliver similarly clinically meaningful outcomes with dapagliflozin use for people with HFpEF. This is reflected in the potential biological mechanisms by which SGLT2 inhibitors may affect cardiovascular outcomes and the previous subgroup analyses of CVOTs demonstrating important beneficial outcomes in people in this cohort. There is an absolute need for meaningful drug therapy in this difficult-to-treat group as no current pharmacological therapy is known to improve cardiovascular mortality. Similarly, the EMPEROR-preserved trial will report on heart failure outcomes in people with HFpEF associated with empagliflozin use. However, caution needs to be taken interpreting the results of these studies because of important differences in trial design and planned study outcomes. Moreover, further clinical trial or real-world evidence to evaluate any additive impact of SGLT2 inhibitors with other drugs classes used in people with heart failure and diabetes such as ACE inhibitors or glucagon-like peptide 1 (GLP-1) analogues would be useful. Benefits seen with dapagliflozin may be associated with important personal, clinical and economic implications given the burden of HFpEF. These may include improved quality of life, less frequent hospitalisation, improved financial costs associated with the disease and less frequent development of co-morbidities including diabetes and CKD in this group.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Disclosures
David M Williams has nothing to disclose. Marc Evans received financial support for consultancy from Novartis, Merck Sharp & Dohme Corp. and Novo Nordisk and has served on the speaker’s bureau for Novartis, Lilly, Boehringer lngelheim, Merck Sharp & Dohme Corp., Novo Nordisk, Janssen and Takeda. Marc Evans is also the Editor-in-Chief of Diabetes Therapy.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Footnotes
Digital Features
To view digital features for this article go to 10.6084/m9.figshare.12799982.
References
- 1.Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3:7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014;171:368–376. doi: 10.1016/j.ijcard.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 3.Shah KS, Xu H, Matsouaka RA, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 4.Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang CC, Struthers AD. Targeting the renin-angiotensin-aldosterone system in heart failure. Nat Rev Cardiol. 2013;10:125–134. doi: 10.1038/nrcardio.2012.196. [DOI] [PubMed] [Google Scholar]
- 6.Safi S, Korang SK, Nielsen EE, et al. Beta‐blockers for heart failure. Cochrane Database Syst Rev. 2017;11:CD012897. [DOI] [PMC free article] [PubMed]
- 7.Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev. 2015;20:193–201. doi: 10.1007/s10741-014-9453-8. [DOI] [PubMed] [Google Scholar]
- 8.Rosano GM, Vitale C, Seferovic P. Heart failure in patients with diabetes mellitus. Card Fail Rev. 2017;3:52–55. doi: 10.15420/cfr.2016:20:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Echouffo-Tcheugui JB, Xu H, DeVore AD, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: findings from Get with The Guidelines-Heart Failure registry. Am Heart J. 2016;182:9–20. doi: 10.1016/j.ahj.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Meagher P, Adam M, Civitarese R, Bugyei-Twum A, Connelly KA. Heart failure with preserved ejection fraction in diabetes: mechanisms and management. Can J Cardiol. 2018;34:632–643. doi: 10.1016/j.cjca.2018.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Lazar S, Rayner B, Campos GL, McGrath K, McClements L. Mechanisms of heart failure with preserved ejection fraction in the presence of diabetes mellitus. Transl Metab Syndr Res. 2020;3:1–5. [Google Scholar]
- 12.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–725. doi: 10.2337/dc16-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e014908. doi: 10.1161/JAHA.119.014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Eng J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 15.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 16.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 17.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 18.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 19.Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV) Am Heart J. 2018;206:11–23. doi: 10.1016/j.ahj.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 20.American College of Cardiology. Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial-VERTIS CV. 2020. https://www.acc.org/latest-in-cardiology/clinical-trials/2020/06/16/11/24/vertis. Accessed 11 Aug 2020.
- 21.Clark AL, Kalra PR, Petrie MC, Mark PB, Tomlinson LA, Tomson CR. Change in renal function associated with drug treatment in heart failure: national guidance. Heart. 2019;105:904–910. doi: 10.1136/heartjnl-2018-314158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams DM, Nawaz A, Evans M. Renal outcomes in type 2 diabetes: a review of cardiovascular and renal outcome trials. Diabetes Ther. 2020;11:369–386. doi: 10.1007/s13300-019-00747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamargo J. Sodium-glucose cotransporter 2 inhibitors in heart failure: potential mechanisms of action, adverse effects and future developments. Eur Cardiol. 2019;14:23–32. doi: 10.15420/ecr.2018.34.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 25.Hwang I, Cho G, Yoon YE, et al. Different effects of SGLT2 inhibitors according to the presence and types of heart failure in type 2 diabetic patients. Cardiovasc Diabetol. 2020;19:69. doi: 10.1186/s12933-020-01042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528–2536. doi: 10.1161/CIRCULATIONAHA.119.040130. [DOI] [PubMed] [Google Scholar]
- 27.Mosenzon O, Wiviott SD, Cahn A, et al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol. 2019;7:606–617. doi: 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 28.Raz I, Wiviott SD, Yanuv I, et al. Effects of dapagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes: a predefined analysis from the DECLARE-TIMI 58 randomised, placebo-controlled trial. Diabetes. 2019;68:244-OR. doi: 10.2337/db19-244-OR. [DOI] [Google Scholar]
- 29.Food and Drug Administration (FDA). FDA approves new treatment for a type of heart failure. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 23 July 2020.
- 30.Heerspink HJL, Stefansson BV, Chertow GM, et al. Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020;35:274–282. doi: 10.1093/ndt/gfz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Packer M, Butler J, Filippatos GS, et al. Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019;21:1270–1278. doi: 10.1002/ejhf.1536. [DOI] [PubMed] [Google Scholar]
- 32.Anker SD, Butler J, Filippatos GS, et al. Evaluation of the effects of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR-Preserved Trial. Eur J Heart Fail. 2019;21:1279–1287. doi: 10.1002/ejhf.1596. [DOI] [PubMed] [Google Scholar]
- 33.ClinicalTrials.gov. Dapagliflozin Evaluation to Improve the LIVEs of Patients With PReserved Ejection Fraction Heart Failure. (DELIVER). 2020. https://clinicaltrials.gov/ct2/show/NCT03619213. Accessed 23 July 2020.
- 34.Inzucchi SE, Docherty K, Kober L, et al. 271-OR: ADA Presidents’ select abstract: effect of dapagliflozin on the incidence of diabetes: a prespecified exploratory analysis from DAPA-HF. Diabetes. 2020;69:271-OR. doi: 10.2337/db20-271-OR. [DOI] [Google Scholar]
- 35.Williams DM, Evans M. 271-OR: ADA Presidents’ select abstract: effect of dapagliflozin on the incidence of diabetes: a prespecified exploratory analysis from DAPA-HF. Diabetes Ther. 2020;11:1925–1934. doi: 10.1007/s13300-020-00889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004-2016. BMC Cardiovasc Disord. 2018;18:74. doi: 10.1186/s12872-018-0815-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Savira F, Wang BH, Kompa AR, et al. Cost-effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol. 2020. 10.1177/2047487320938272. [DOI] [PubMed]
- 38.Norhammar A, Bodegard J, Nyström T, et al. Dapagliflozin vs non-SGLT-2i treatment is associated with lower healthcare costs in type 2 diabetes patients similar to participants in the DECLARE-TIMI 58 trial: a nationwide observational study. Diabetes Obes Metab. 2019;21:2651–2659. doi: 10.1111/dom.13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santas E, Valero E, Mollar A, et al. Burden of recurrent hospitalizations following an admission for acute heart failure: preserved versus reduced ejection fraction. Rev Esp Cardiol (Engl Ed) 2017;70:239–246. doi: 10.1016/j.recesp.2016.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.


