Figure 1.
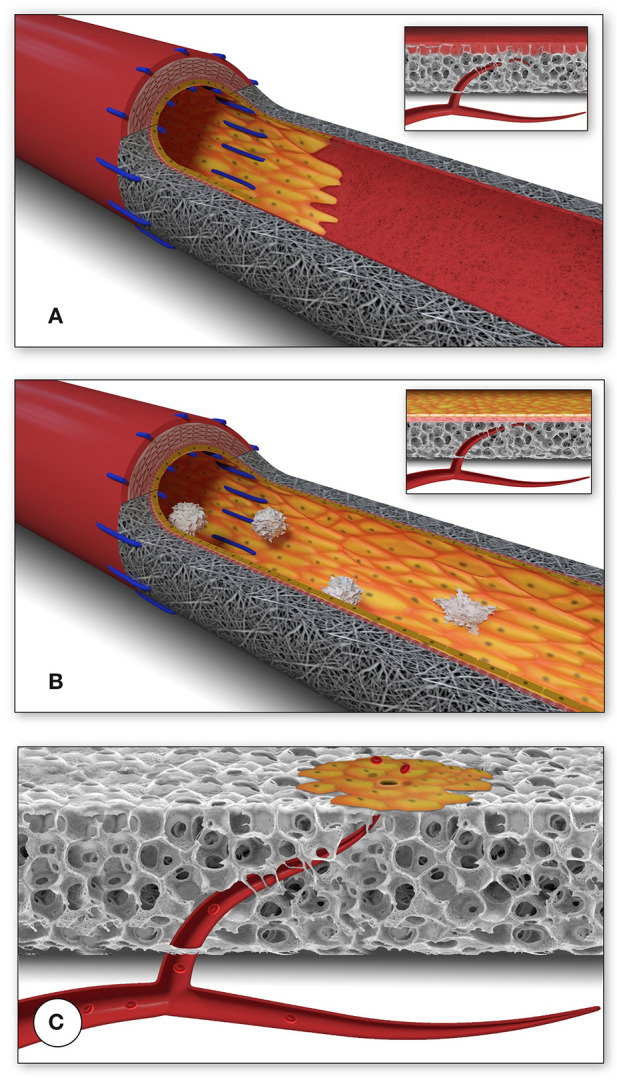
Schematic presentation of the fundamental difference in cellularization and healing of prosthetic cardiovascular implants in humans (A) and in animal models (B). In humans, transanastomotic outgrowth hardly exceeds a few millimeters even after years of implantation. Continual fibrinogen and platelet replenishment from the blood leads to a compacted surface thrombus in the luminal interstices of the scaffold that increasingly becomes hostile toward capillary penetration similar to the wall thrombus of aneurysms. Over time, this compacted acellular material in the luminal layers of a scaffold becomes prohibitive for transmural endothelialisation (Insert A) even if scaffold structure and/or degradability would facilitate capillary penetration. The rapid trans-anastomotic outgrowth of adjacent endothelium and its subintimal cells in the vast majority of animal models (B) also mitigates transmural vascularization (Insert B) while actively recruiting cells from the circulation. As such, the entire healing pattern in most animal models from surface endothelium to intramural cell population is non-predictive for the tissue response in patients. For transmural endothelialisation to be successful (C), models need to be chosen where the presence of a surface endothelium is not pre-empted. Only this allows to study the antagonistic dynamics between ingrowth spaces, accelerated angiogenesis and the build-up of increasingly impenetrable, compacted thrombus in the luminal interstices of a scaffold.
