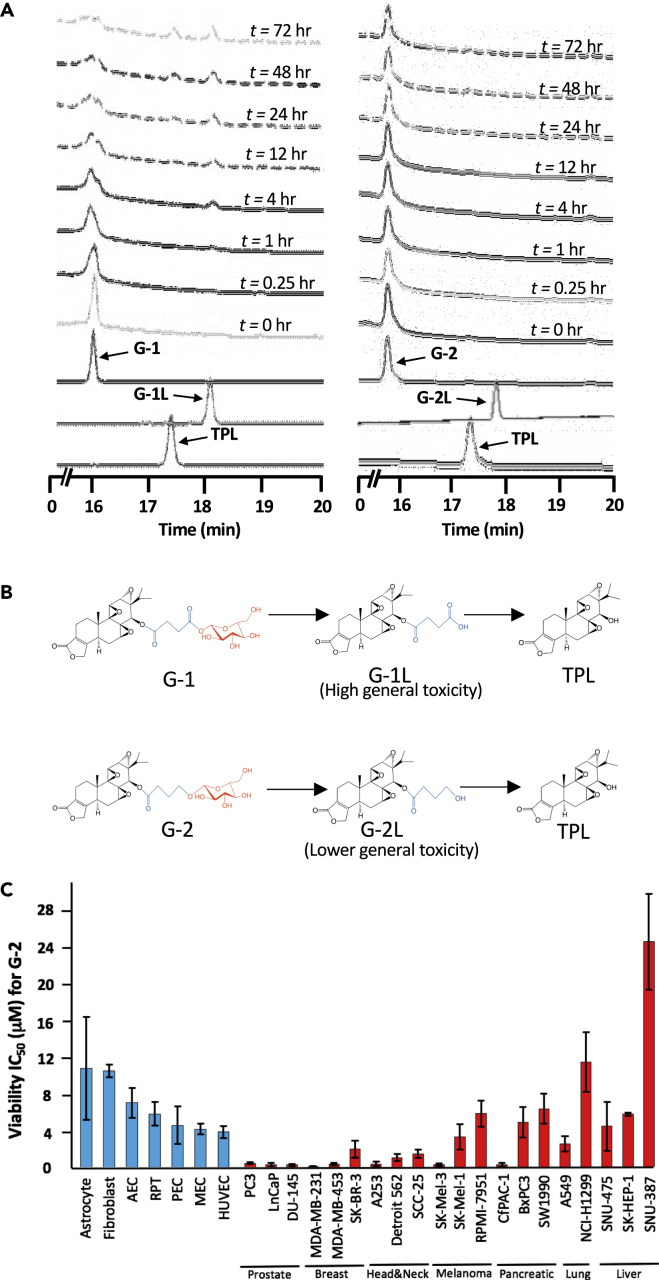
Figure 2.
Glutriptolide-2 Possesses Increased Stability in Human Serum and Lower General Toxicity toward Nonmalignant, Primary Cells Relative to G-1
(A) Hydrolysis of G-1 and G-2 at different incubation times in human serum as monitored by tandem HPLC-MS. Chromatograms were taken at A218.
(B) Chemical structures of G-1 and G-2 with hydrolysis intermediates G-1L and G-2L that subsequently releases triptolide (TPL). Structural motifs in glutriptolides are highlighted (black = triptolide, blue = linker, and red = glucose).
(C) Primary cell viability as measured by XTT assay exhibits reduced sensitivity to G-2 in comparison to multiple cancer cell lines. Liver, lung, melanoma, and pancreatic cancer cell lines respond poorly to G-2 treatment. HUVEC = Human Umbilical Vascular Endothelial Cell, MEC = Mammary Epithelial Cell, PEC = Prostate Epithelial Cell, RPT = Renal Proximal Tubule, AEC = Airway Epithelial Cell. Data are represented as mean ± SEM viability relative to DMSO (n = 3–7).