Figure 4.
Glutriptolide-2 Induces Apoptosis Signaling
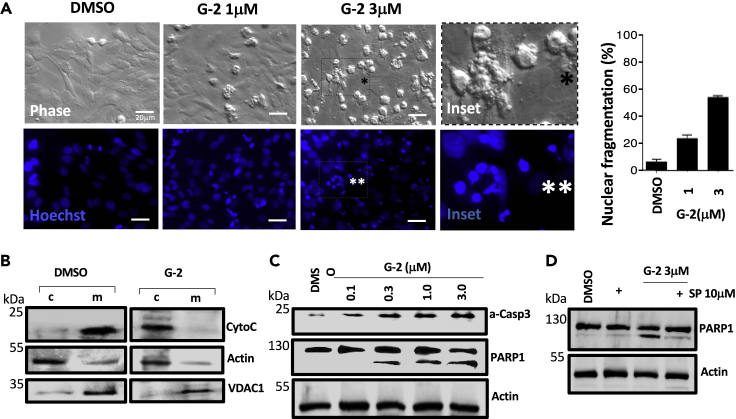
(A) Bright phase micrographs show minimal cytopathology with DMSO exposure in contrast to G-2 treatments especially with 3 μM G-2 where numerous cells round up and bleb (inset with black asterisk). Nuclear fragmentation, as detected by cytochemical analysis using Hoechst 33258 stain, in round up HeLa cells is dramatically increased by G-2 treatment (inset with two white asterisks) but not in DMSO. Data are represented as percentage of nuclear fragmented cells relative to total cells ±SE (n = 3).
(B) Cytochrome c release during G-2 treatment was assessed by centrifugal separation of mitochondria followed by western blot analysis using cytochrome-c-specific antibody. Exposure of HeLa cells to 3 μM G-2 triggers the release of cytochrome c from the mitochondria (m) to the cytosol (c). Actin- and VDAC1-specific antibodies were used to ensure the efficiency of cytoplasm and mitochondria fractionation, respectively.
(C) Western blot analysis of whole cell lysates for active caspase 3 (a-Casp3) and PARP1 during G-2 treatment shows a dose-dependent increase in caspase 3 activation. Pronounced PARP1 cleavage by active caspase 3 is also observed with increasing concentrations of G-2.
(D) Degradation of XPB in cells by 10 μM sprironolactone dampens G-2-induced apoptosis signaling as indicated by reduced PARP1 cleavage in whole cell lysates subjected to western blot analysis. Actin was used as loading control. Scale bar, 20 μm.