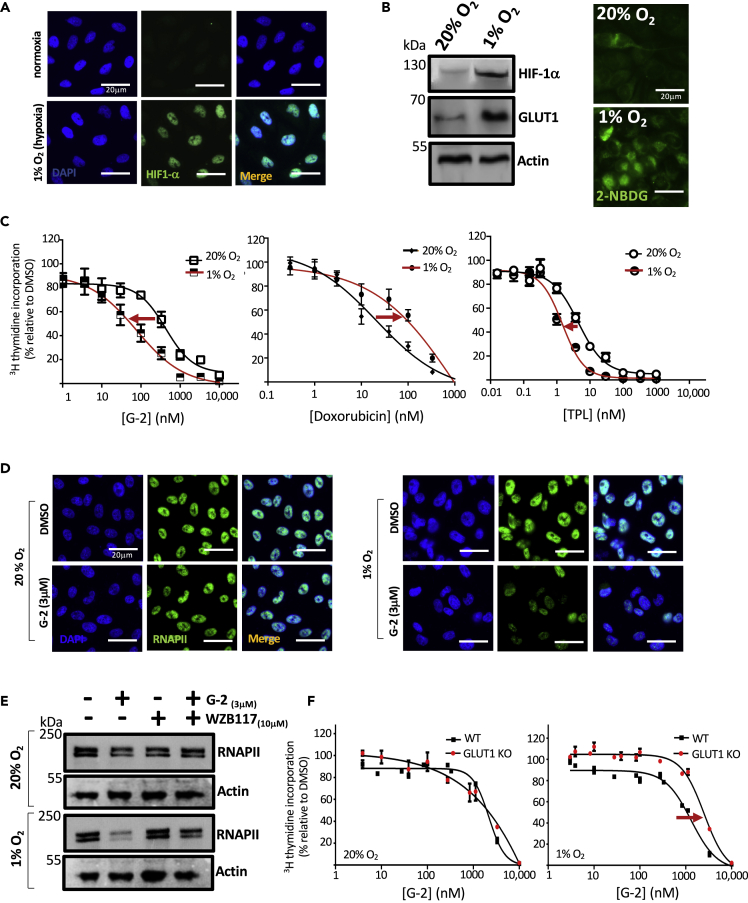
Figure 6.
Hypoxia Enhances Antiproliferative Effect of Glutriptolide-2
(A) Immunocytochemical analysis of fixed cells using antibodies specific to HIF-1α show that exposure to hypoxia (1% O2) for 24 h stabilizes endogenous HIF-1α compared with normoxia (20% O2) in PC3 cells.
(B) Western blot analysis of whole cell lysates for endogenous HIF-1α shows an increase during hypoxia compared with normoxia, which also corresponds with an increase in glucose transporter 1 (GLUT1).
(C) Hypoxia enhances the antiproliferative effect of G-2 at 48 h posttreatment as measured by 3H thymidine incorporation, whereas co-treatment with doxorubicin and hypoxia reduces drug potency. Triptolide (TPL) shows a modest antiproliferative effect. Data are represented as mean ± SE relative to DMSO (n = 3).
(D) Immunocytochemistry using antibody specific to RPB1 shows that exposure of cells to hypoxia triggers an early onset of RNAPII subunit RPB1 degradation by 3 μM glutriptolide-2 after 6 h.
(E) Whole cell lysates subjected to western blot using anti-RPB1-specific antibody shows that 10 μM glucose transporter 1 inhibitor WZB117 antagonizes the early onset of RNAPII degradation triggered by 3 μM G-2 and hypoxia.
(F) DLD-1 WT cells exposed to hypoxia exhibited enhanced sensitivity to G-2 in comparison to DLD-1 GLUT1 knockout (GLUT1 KO) cells. No difference in sensitivity is observed between DLD-1 WT and GLUT1 KO under normoxia. Data are represented as mean ± SEM relative to DMSO (n = 3). Scale bar, 20 μm.