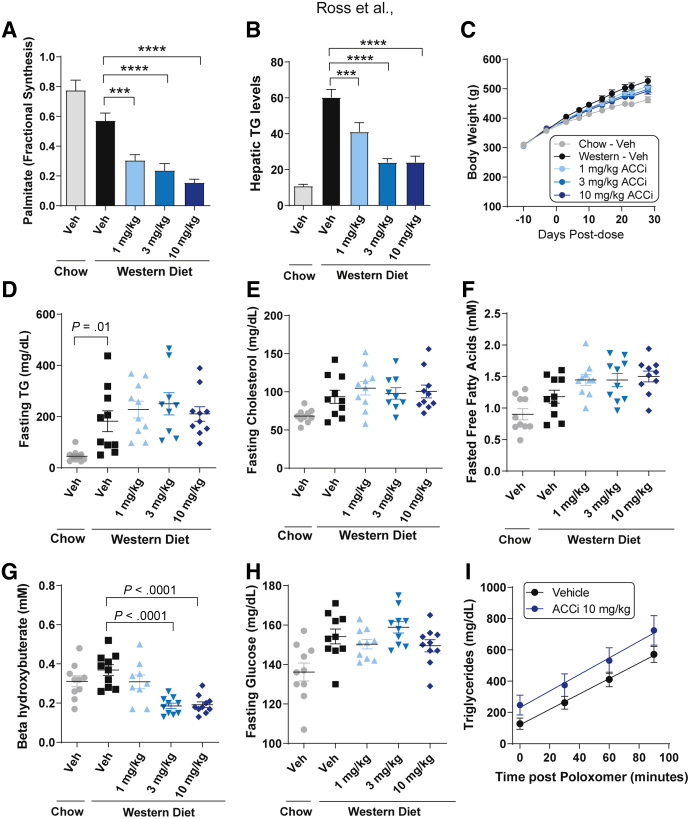
Figure 4.
Effect of PF-05221304 on fractional hepatic de novo lipogenesis, steatosis, and metabolic parameters in Western diet–fed rats. (A and B) Once-daily oral administration of PF-05221304 for 4 weeks (A) suppresses hepatic DNL as assessed by deuterated water incorporation into lipids and (B) ameliorates hepatic steatosis in a dose-dependent manner. (C) Body weight, (D) fasting plasma TG, (E) cholesterol, (F) free fatty acids, (G) β hydroxybutyrate, and (H) glucose are shown. n = 10 animals per treatment group. (I) A poloxamer challenge was performed on a parallel cohort of animals to assess effects on hepatic VLDL-TG secretion. n = 6–8 per group. ∗∗∗P < .001 relative to vehicle. ∗∗∗∗P < .0001 relative to vehicle. ACCi, PF-05221304; Veh, vehicle.