Abstract
The inflammatory response is a complex, tightly regulated process activated by tissue wounding, foreign body invasion, and sterile inflammation. Over the decades, great progress has been made to advance our understanding of this process. One often overlooked aspect of inflammation is its sequel: resolution. We know that dysregulated resolution often results in numerous chronic degenerative diseases such as arthritis, cancer, and asthma. However, identification of components and mechanisms of resolving pathways lags behind those of proinflammatory processes, yet represents overlooked therapeutic opportunities. One approach is identification of endogenous, negative compensatory mechanisms, which are activated in response to inflammation for the purpose of resolution of that inflammatory stimuli. This review will focus on literature that describes expression and function of interleukin-19, a proposed anti-inflammatory cytokine, in numerous inflammatory diseases. The literature concerning IL-19 is complex, context-dependent, and often contradictory. The expression and function of IL-19 in the inflammatory response are in no way settled. We will attempt to clarify the role that this interesting and understudied cytokine plays in resolution of inflammation and discuss its mechanisms of action in different cell types. We will present a hypothesis that endogenous IL-19 expression in response to inflammatory stimuli is a cellular compensatory mechanism to dampen inflammation. We further present studies suggesting that while endogenously expressed IL-19 may be a response to inflammation, pharmacological levels may be necessary to effectively resolve the inflammatory cascade.
Keywords: FXR1, inflammation, interleukin-19, mRNA stability, resolution
INTRODUCTION
Inflammation and Its Resolution
Inflammation is a highly coordinated series of molecular and cellular events leading to elimination of the foreign invader, repair of damaged tissue, and more long term, the promotion of adaptive immune memory. An effective inflammatory response relies upon intricate cellular and molecular interactions between the immune system, the vascular system, and the affected tissue. One inflammatory response is production of soluble mediators by immune cells, including neutrophils, monocytes, tissue macrophages, lymphocytes, and mast cells, and nonimmune cells, including fibroblasts and vascular endothelial cells (VECs). These soluble factors include cytokines and chemokines, complement, and prostaglandins. An immediate early response to inflammation is the NF-κB-mediated upregulation of adhesion molecules on VECs and circulating leukocytes. This in turn promotes extravasation of leukocytes from the circulation into the affected tissue, leading to localized inflammation. Guided by a chemokine gradient, arriving immune cells respond to the inflammatory stimulus in a multitude of ways, including phagocytosis of invading microorganisms and damaged host cells, and begin the repair process of tissue remodeling and angiogenesis (44).
Ideally, inflammation is a self-limiting, protective process. Resolution is not merely a cessation of inflammatory signals, but like inflammation, is a tightly regulated balance of cellular and molecular events in its own right (5). For inflammation to properly resolve, the presence of a dynamic, endogenous anti-inflammatory, resolving pathway must be activated to not only inhibit factors that drive inflammation, but also induce wound resolution to return the tissue to its original state. Each of the events listed in the preceding paragraph, activation of NF-κB, transcription and synthesis of soluble mediators and adhesion molecules, and leukocyte extravasation represent processes that are targeted for resolution. A standing-down of the inflammatory response is activated once the proinflammatory stimuli is eliminated; this is not a passive process (20). An initial switch off of NF-κB transcriptional processes coupled with posttranscriptional destabilization of mRNA rapidly halts synthesis of proinflammatory mediators in a tightly regulated, controlled process. Together, these events will decrease leukocyte recruitment, clear existing leukocytes by egress from the tissue or efferocytosis in place, and promote healing of tissue damage by repair of blood vessels and synthesis of capillaries and lymphatics. This is a tightly regulated process involving multiple, exquisitely regulated signaling pathways requiring harmonious balance between proinflammatory and anti-inflammatory mediators (18). An altered balance of these “opposing forces” can skew the outcome of the inflammatory process and lead to chronic inflammation and subsequent tissue damage. Therefore, expression of endogenous anti-inflammatory factors is an essential compensatory event used by organisms to limit the severity and duration of inflammation. One promising approach to resolve inflammation is to increase abundance of compensatory factors above endogenous levels to pharmacological concentrations. In this review, we propose that expression of one such endogenous factor, the cytokine interleukin-19, is a negative compensatory response to resolve inflammation. In this review, after a description of IL-19, we present IL-19 effects on polarization of adaptive immunity, and then illustrate how IL-19 attenuates inflammatory mRNA transcript stability in nonimmune cells.
IL-19: a Multifactorial Anti-Inflammatory Interleukin
Interleukin-19 was first described in 2000 and based on its sequence and similar crystal structure, it was initially considered to be an IL-10 family homolog. Subsequent studies have placed IL-19 into an IL-20 subfamily containing IL-20, IL-22, and IL-24, based on receptor usage (36, 46). Each of these interleukins signals through a receptor complex consisting of a heterodimer of the IL-20Rα and IL-20Rβ chains. Although both IL-20 and IL-24 can signal through a heterodimer composed of the IL-22Rα and IL-20Rβ chains, IL-19 cannot (for an excellent review, see Ref. 60). IL-19’s pleiotropic effects can likely be attributed to the cell-type distribution of these receptor chains (6, 58). In this regard, cytokine-inducible expression of IL-20β receptor chain subunits can result in major differences in IL-19 function between different cell types (32). Regardless of receptor complex usage, IL-20 family members activate the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway, and importantly, STAT3 (7). IL-19 was first described as expressed in human monocytes, and basal levels of IL-19 can also be detected in human B and T lymphocytes. IL-19 can be upregulated in monocytes, B and T lymphocytes by lipopolysaccharides (LPS) and granulocyte colony-stimulating factor stimulation, and can be induced in these cells by IL-4 and IL-13 only when they are pretreated with LPS. In nonimmune cells, basal levels of IL-19 are not detected, but can be induced by TNFα and other inflammatory stimuli (43, 57). There are some reports of IL-19 participation in proinflammatory responses, including psoriasis, asthma, and some cell culture models. In many respects, IL-19 may indeed exert pleiotropic effects depending on the cell type and/or disease model. Many conclusions pertaining to IL-19 being proinflammatory or anti-inflammatory, particularly in earlier studies, were drawn on the basis of observations that IL-19 expression was increased in a particular inflammatory situation. In some circumstances it was not taken into consideration that IL-19 expression was increased in response to the inflammatory insult as part of a compensatory pathway to resolve inflammation. Nevertheless, IL-19 is considered to be a Th2 interleukin because it promotes the Th2, rather than the Th1 (T helper) response in lymphocytes, although the mechanism that drives this response has not yet been fully characterized (24, 46, 48). IL-19 is most often considered to be an anti-inflammatory cytokine in immune cells for the following reasons: 1) IL-19 treatment of maturing antigen-presenting cells promotes the Th2 (regulatory) T-cell response, rather than the Th1 (T helper), response. This Th2/Th1 balance shifts the phenotype toward a more anti-inflammatory phenotype; 2) IL-19 treatment increases IL-4 and decreases IFNγ in regulatory T cells; 3) IL-19 stimulation of peripheral blood mononuclear cells (PBMCs) induces IL-10 secretion of these cells; 4) IL-19 can inhibit production of IgG from B cells (31, 43, 46, 48). IL-19 anti-inflammatory pathways are multifactorial, with resolving effects on each of the proinflammatory events described in the opening paragraph. In inflammatory cells such as T cells and macrophages, IL-19 can polarize adaptive immunity to the Th2 and M2 phenotype, respectively. In both immune and nonimmune cells, IL-19 can reduce synthesis of proinflammatory cytokines. In endothelial cells, IL-19 can reduce adhesion molecule expression leading to reduction in leukocyte infiltration as well as induce angiogenesis leading to resolution of tissue damage and repair of blood vessels. This is summarized in Fig. 1.
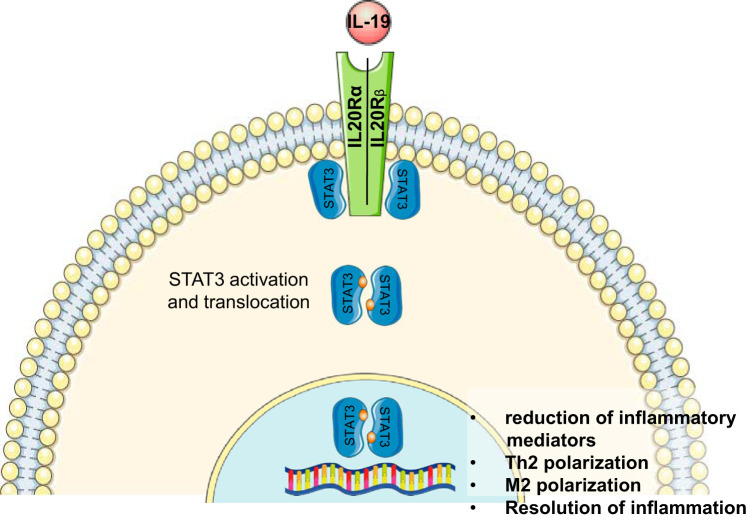
Fig. 1.
Proposed pathway of IL-19 resolution of inflammation in immune cells. IL-19 receptor engagement activates STAT3, which mediates anti-inflammatory signaling. Inflammatory gene expression is reduced, leading to T helper 2 (Th2) polarization in lymphocytes, and M2 polarization in macrophages.
RESOLUTION OF INFLAMMATION IN IMMUNE CELLS
IL-19 Polarizes Adaptive Immunity to an Anti-Inflammatory Phenotype
In T cells, the Th2 arm of adaptive immunity tends to limit the magnitude of the inflammatory response. Initial studies of IL-19 focused on lymphoid cells and strongly pointed to a role for IL-19 in induction of Th2 responses. Other earlier studies in human monocytes indicated that IL-19 decreased IFNγ expression, and the Th2 cytokines IL-4 and IL-13 increased IL-19 expression, leading to an IL-19-induced polarized maturation of T cells to the Th2 phenotype (24). IL-19 induced expression of the prototypical Th2 interleukin, IL-10, in human PBMCs (31). In another study, IL-19 polarized T cells to the Th2 phenotype by both a downregulation of IFNγ and an upregulation of IL-4 and IL-13 (46).
An excellent example of a localized as well as systemic inflammation is atherosclerotic vascular disease. Atherosclerosis is a vascular inflammatory disease involving numerous cell types and a multitude of proinflammatory mediators including cytokines, adhesion molecules, and growth factors (for an excellent recent review, see Ref. 39). Attenuation of global inflammatory responses can reduce severity of vascular disease, as recently demonstrated by the groundbreaking CANTOS (Canakinuab Anti-Inflammatory Thrombosis Outcomes Study) clinical trial (51). A number of in vivo and ex vivo studies demonstrated vascular protection as a secondary effect of IL-19-mediated immune polarization. Initially, it was observed that IL-19 expression in numerous cell types in coronary arteries was significantly increased in symptomatic, compared with asymptomatic patients with coronary artery disease (14). This was counterintuitive for a presumed anti-inflammatory cytokine, and drove the hypothesis that increased IL-19 expression was a compensatory mechanism to resolve chronic vascular inflammation. A number of complementary murine models were used to test this hypothesis. Atherosclerosis-prone, low-density lipoprotein receptor (LDLR) knockout mice fed an atherogenic high-fat diet (HFD) are polarized to Th1 since hyperlipidemia is proinflammatory (19, 53). In one study in which mice were injected with rmIL-19, in addition to significantly attenuated atherosclerosis, gene expression in splenocytes from rmIL-19-injected mice demonstrated increased expression of T-cell Th2 polarization markers, including GATA3 and FoxP3, with a concomitant decrease in Th1 T-cell markers, including IFNγ, IL-1β, and IL-12β (14). Polarization was not limited to lymphocytes, as in a second study, plaque and spleen from rmIL-19-injected LDLR knockout mice demonstrated increased macrophage M2 marker expression, including Arg1 and Ym1, with a corresponding decrease in the in M1 marker Arg2 (21). IL-19 expression was increased in primary human macrophages cultured with the M2-polarizing cytokines IL-4 and IL-13 (21). In a more controlled approach, bone marrow-derived macrophages (BMDM) from wild-type mice cultured with IL-19 exhibited decreased TNFα-driven expression of IL-12β, MCP1, and TNFα mRNA (21). The LDLR knockout mouse, a commonly used mouse model that develops atherosclerosis when fed a high-fat, Western-type diet, was crossed with the IL-19 knockout mouse (49). The IL-19/LDLR double-knockout (DKO) mouse developed significantly more atherosclerotic plaque compared with control mice. In addition, these mice had increased global inflammatory responses, including increased abundance of proinflammatory cytokines and Th1 markers including Tbet, Arg2, IL-12β, and RORγ, and increased TNFα, IL-1β, and MCP-1 mRNA in the spleen and aortic arch, respectively. Importantly, when these DKO mice were injected with rmIL-19, the atherosclerotic phenotype was “rescued,” with reduced atherosclerosis, and expression of proinflammatory cytokines and Th1 markers reduced in the spleen in these mice, tightly linking IL-19 activity with inflammatory resolution. Similar effects on macrophage M2 polarity were observed in wild-type mice injected with rmIL-19 and subject to hindlimb ischemia surgery (50). Collectively, these studies strongly suggest that at least in a vascular inflammation model, the increased expression of IL-19 observed may represent a cellular event to resolve chronic inflammation.
In another example of IL-19 dampening of global immune response, human patients with spondylarthritis (SpA), a systemic inflammatory disease affecting multiple sites, had decreased levels of IL-19 in plasma and synovial fluid compared with control subjects (35). PBMCs and synovial fluid mononuclear cells isolated from SpA patients produced less IL-19 when stimulated with LPS. The authors of this study concluded that endogenously produced IL-19 may dampen the global immune response, and in patients with reduced IL-19 may lead to systemic inflammation. The therapeutic value of IL-19 was assessed in protection of mice from myocardial damage postinfarction (1) where it was found that treatment of mice with rmIL-19 preserved normal cardiac function. The authors concluded that one mechanism for this protection was a reduction in inflammatory infiltrate. Of the macrophages that did infiltrate, the proportion of M1 was reduced, and M2 was increased. Two studies indicated that in an inflammatory bowel disease model in which both innate and adaptive immune responses participate, IL-19-deficient mice were more susceptible to acute colitis compared with controls (2). Macrophages isolated from these mice demonstrated a concomitant increased expression of proinflammatory cytokines such as IL-1β, IFNγ, IL-6, IL-12, and TNFα when stimulated with LPS. A similar model utilized by the same group observed that the absence of IL-19 also increased expression of IL-1β and also decreased abundance of IL-4-producing T lymphocytes (41). When taken in total, the protection afforded by IL-19 in a number of disease models was imparted, at least partially, by polarization of adaptive immunity from a Th1 and M1 proinflammatory, to a Th2 and M2 anti-inflammatory phenotype. They imply that physiological levels of IL-19 may not be sufficient to effectively reduce inflammation, but pharmacological levels may be necessary to resolve the inflammatory cascade. These molecular and cellular events are summarized in Fig. 2.
Fig. 2.
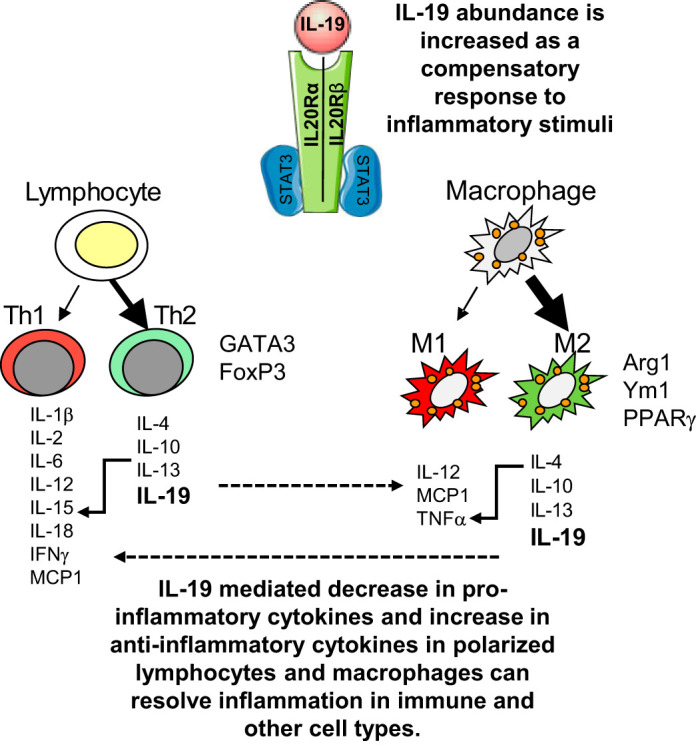
Effect of IL-19 on polarization of adaptive immunity. IL-19 expression is increased by inflammatory stimuli, presumably as a negative, compensatory mechanism. In an inflammatory context, increased IL-19 signaling results in polarization of lymphocytes to the T helper 2 (Th2) phenotype, with a concomitant decrease in proinflammatory cytokines, and an increase in Th2-associated transcription factors and anti-inflammatory cytokines. Upon IL-19 stimulation, macrophages are polarized to the M2 phenotype, with an increase in M2-associated transcription factors and anti-inflammatory cytokines. The cross talk between polarized immune and other cell types results in IL-19-mediated protection and inflammatory resolution in a number of disease models.
IL-19 Is Neuroprotective
IL-19 has been implicated in resolution of inflammation in neurological disorders and neuroinflammation. Murine astroglial cells express IL-19 in response to LPS, yet IL-19 is able to dampen inflammatory cytokine expression in these cells, the authors suggesting that IL-19 is part of a regulatory loop to resolve inflammatory responses in these cells (45). In a second investigation, IL-19 attenuated bacterial or Toll-like receptor ligand-stimulated proinflammatory cytokine production in astrocytes (8). IL-19 deficiency increased production of IL-6 and TNFα in activated microglia, and IL-19 treatment suppressed expression of these cytokines in these cells (29). Wild-type mice in which IL-19 was injected were subject to transient cerebral artery occlusion to induce stroke. Cerebral infarcts in IL-19-treated mice were significantly smaller compared with control mice. In this model, IL-19 reduced inflammatory cytokine expression in microglia, macrophage, and T-lymphocytes, and reduced neutrophil activation (63). Thus, systemic administration of IL-19 globally dampened the inflammatory response to tissue damage as well as directly dampening the neuronal response to injury.
STAT3 Is the Initial IL-19 Effector Protein in Leukocytes
While it is evident that IL-19 polarizes immune cells, the mechanism is not yet clarified. One clue may come from IL-19 receptor usage. IL-19 signals through the IL-20Ra and IL-20Rb receptor complex, and in myeloid cells, rapidly activates STAT3 (24). This is revealing, as it has been posited that Th2 signaling is mediated by IL-20Rb, as knockout of this receptor chain polarizes T-cell responses to Th1-proinflammatory type responses (58). Numerous reports indicate that IL-19 induces STAT3 phosphorylation and nuclear translocation (12, 47). In leukocytes, STAT3 can mediate both proinflammatory and anti-inflammatory signaling, even in the same cell type (30), with the caveat that almost all of the conclusions of anti-inflammatory regulation were drawn from cells stimulated with IL-10 (42, 61). Importantly, STAT3 deficiency in macrophages and neutrophils leads to enhanced Th1 responses (56). Other less direct lines of evidence show that STAT3 mediates anti-inflammatory signaling, as mutations in STAT3 are associated with immune alterations such as autoimmunity and cancer (28).
IL-19 ANTI-INFLAMMATORY EFFECTS ON NONIMMUNE CELLS
Asthma Is a Th2-Driven Disease
IL-19 expression in cells of the respiratory system may also represent a compensatory, or immune-resolving event. While initial studies focused on IL-19 as a direct immune modulator, several in vivo and ex vivo studies suggest that IL-19 has potent anti-inflammatory effects on cells outside the immune system. Asthma is a chronic inflammatory disease of the airway, with symptoms of breathing difficulty causing airway narrowing and obstruction. Asthma is also characterized as a dysregulated Th2 disease (23, 59), as the prototypical Th2 cytokines, IL-4, IL-5, and IL-13, are secreted in allergic asthma (40, 52). Following response to allergens, IL-19 expression is also elevated in the lungs and serum of wild-type mice (38). This same study also found that plasma IL-19 levels were increased in asthmatic children. In a related study, bronchial epithelial cells in airway from asthmatic and patients with chronic obstructive pulmonary disease (COPD) express large amounts of IL-19. These same cell types responded to asthma mediator cytokines, particularly IL-13, which is known to drive asthma, to induce large amounts of IL-19 expression (40, 64), further suggesting a complex regulatory feedback loop, in that IL-19 expression in this disease may be a compensatory mechanism to reduce pulmonary inflammation. On the other hand, since a Th2-dominant immune response is characteristic of asthma, and IL-19 is a driver of Th2 cytokine production, we need to entertain the possibility that IL-19 expression in the context of this disease may be deleterious.
IL-19 Induces Keratinocyte Proliferation
IL-19 expression may be increased in epidermal cells as a compensatory event in response to psoriatic inflammation. IL-19 and its receptor complex are highly expressed in keratinocytes, and expression of IL-19 is especially increased in psoriatic skin. Nevertheless, it appears that IL-19 function is complex and not completely understood. Many conclusions pertaining to IL-19 function were drawn on the basis of observations of increased IL-19 expression in keratinocytes in inflammatory situations such as psoriasis. However, it was not considered that increased IL-19 expression could be part of a compensatory pathway to resolve psoriatic inflammation. Two main inducers of IL-19 mRNA expression in keratinocytes are IL-17 and keratinocyte growth factor (KGF). IL-19 expression is increased with severity of psoriasis, and a decrease in IL-19 correlates with a decrease in epidermal hyperplasia (33). Interestingly, this report and other studies (55) implicate IL-19 expression as part of a feedback loop in which IL-19-stimulated keratinocytes produce KGF leading to keratinocyte proliferation, and further expression of IL-19, suggesting a role in wound repair. IL-19 amplified many effects of IL-17A stimulation on keratinocytes, including the induction of β-defensins, and T-helper type 17-cell- and neutrophil-attracting chemokines (62). That being said, while the actual role of IL-19 as a protagonist of psoriasis and epidermal inflammation remains unclear, at this junction one certainty is that IL-19 expression can be considered part of the IL-23/IL-17 axis, which is known to drive psoriasis. At the very least, IL-19 expression can serve as a biomarker for the activity of the IL-23/IL-17 axis that drives chronic inflammatory epidermal disorders.
Regulation of mRNA Stability Is a Mechanism for Resolving Inflammation
Modulation of mRNA stability is a potent, though oft overlooked posttranslational mechanism for resolving inflammation. An estimated 5–8% of all human transcripts contain AU-rich elements (AREs), which consist of various large clusters of overlapping AUUUA pentamer and UUAUUUAUU monomers (3). The ARE represents a binding site for RNA-binding proteins (RBPs), which often regulate the translation or half-life of the transcript by positive or negative regulation of its stability. ARE-mediated regulation of transcripts provides highly sensitive and specific control of mRNA abundance. Importantly, many reports determined that RNA stability is a major mechanism for homeostasis of global inflammation, and many proinflammatory genes are targeted for degradation by cis-acting AREs in their 3′-untranslated regions (UTR) (4, 26). Exchange or insertion of AREs from unstable transcripts can decrease the stability of normally stable transcripts (54). More germane for a discussion of inflammatory resolution, the half-life of TNFα mRNA, which is normally unstable, can be increased by mutation of ARE in its 3′-UTR. Furthermore, mice that lacked the TNFα ARE completely had increased circulating TNFα and a proinflammatory phenotype (34). It was reported that the stability of TNFα mRNA was more a driver of inflammatory gene expression than transcriptional control elements (2). Together, regulation of mRNA stability has been posited as an underutilized therapeutic opportunity (11), indicating that cytokines that can reduce mRNA stability can effectively resolve an inflammatory response.
IL-19 Can Reduce Inflammatory Gene Expression by Reducing mRNA Stability
A picture has emerged over the past several years that in addition to polarization of adaptive immunity, IL-19 can, in effect, “polarize” nonimmune cells to a Th2-like, anti-inflammatory phenotype, mediated at least partially by polarization of inflammatory mRNA stability. Human R Antigen (HuR) is recognized as one of the best characterized RBPs and is the major mRNA stability protein (13). HuR stabilizes a multitude of ARE-containing mRNAs, including those coding for cytokines, adhesion molecules, growth factors, and other proinflammatory transcripts. HuR is ubiquitously expressed in all cell types, and as a major regulator of ARE-bearing transcripts, it is a key mediator of the inflammatory response in immune and nonimmune cells (11, 16). Several studies in nonimmune cells established that IL-19 can decrease the mRNA stability and protein abundance of ARE-containing, proliferative, and proinflammatory genes in an HuR-dependent mechanism (9, 15, 49, 57). An early study in primary human vascular smooth muscle cells (VSMCs), a cell that comprises the media of blood vessels and comprises the viscera, showed that IL-19 decreased the protein and mRNA abundance of select inflammatory transcripts (9). Unlike other Th2 interleukins such as IL-10 that can reduce inflammatory gene expression, this study showed that IL-19 did not decrease NF-κB expression or activity, prompting the investigators to turn to mRNA stability as a mechanism of IL-19 effects. IL-19 was able to decrease the mRNA stability of ARE-bearing transcripts, but not those that did not contain ARE (9). The ability of HuR to stabilize mRNA corresponds with its translocation from a predominantly nuclear location into the cytoplasm (11), which is logical considering that the cytoplasm is where mRNA is processed. This study showed that IL-19 decreased HuR nucleocytoplasmic shuttling in response to FCS stimulation. A second study in primary human endothelial cells showed an IL-19 mediated a decrease in TNFα-driven HuR nucleocytoplasmic shuttling and an associated reduction in adhesion molecule mRNA stability (15). Although the precise mechanism of how IL-19 prevents HuR translocation has yet to be elucidated, this does point to an NF-κB-independent mechanism to reduce adhesion molecule expression, suggesting a promising posttranscriptional based-therapeutic strategy to resolve inflammation.
An IL-19/HuR axis also directly participates in the reduction of experimental atherosclerosis. In IL-19/LDLR double-knockout mice, HuR protein and mRNA abundance were increased in the spleen and aorta of DKO mice compared with control mice (49). In VSMCs explanted and cultured from DKO mice, TNFα mRNA and unexpectedly, HuR mRNA, stability was also increased, which could be rescued to wild-type levels by adding rmIL-19 to these cells. Together, IL-19 regulation of HuR abundance, and particularly HuR mRNA stability, in addition to reduction in HuR cytoplasmic translocation shows a multifaceted attenuation of HuR activity by IL-19. This also points to a direct, HuR mediated posttranscriptional mechanism for resolution of inflammation in nonimmune cells by reduction in mRNA stability of ARE-containing proinflammatory transcripts.
Two potential mechanisms may be responsible for IL-19 reduction in HuR abundance. miRNAs are emerging as key regulators of inflammatory processes (17). Initially, it was shown that IL-19 stimulation of human VSMCs induced expression of miRNA133a, a muscle cell-specific miRNA (22). Database analysis predicted miR133a to target HuR 3′-UTR, and this investigation showed that miR133a could reduce HuR mRNA and protein abundance in VSMC. Importantly, miR133a expression was detectible in DKO VSMC only at very low levels, but its expression was rescued to wild-type levels with the addition of IL-19, further linking the observed increased HuR in DKO with IL-19 expression. In a second approach, unbiased mass spectrometry was used to identify HuR-interacting proteins in VSMCs (27). Not surprisingly, a number of interacting proteins were identified that functioned in various aspects of mRNA processing. One of them was identified as fragile X mental retardation syndrome-related protein 1 (FXR1), a recognized RBP (25). Not only was this protein identified as an HuR-interacting protein, but, at least in VSMCs, FXR1 expression is induced by IL-19 (27). A variety of knockdown and overexpression experiments identified FXR1 as regulating inflammatory gene mRNA stability in VSMCs.
RBP, which destabilizes mRNA, outnumbers mRNA-stabilizing RBPs, and there appears to be a competitive balance between these two “opposing forces” (37). Through a synthesis of this literature, we propose that mRNA-destabilizing RBPs may be considered as immune modulators to dampen the inflammatory response via AREs. TNFα is considered to be a master inflammatory cytokine as it induces expression of multiple proinflammatory genes, and RNA-electro mobility shift assays (cRNA-EMSAs) using the TNFα 3′-UTR determined that similar to HuR, FXR1 binds RNA via canonical ARE sequences. The addition of RNAse A to coimmunoprecipitation abolished the FXR1-HuR protein-protein interaction, suggesting that FXR1 did not directly interact with HuR, but rather, was tethered on the same mRNA strand, implying that FXR1 competed with HuR for ARE occupancy. Most importantly for this discussion, stimulation of VSMC with IL-19 significantly induces FXR1 mRNA and protein expression. HuR is suspected to autoregulate its expression as it contains ARE elements in its 3′-UTR, which can potentially interact with HuR itself (10). Long-term treatment of VSMC with IL-19 reduces HuR abundance and mRNA stability (14, 27), but these reductions are abolished when FXR1 is knocked down, indicating that IL-19 reduction of HuR requires FXR1. Since FXR1 expression was induced by IL-19 and was also necessary for IL-19 destabilization and reduction of HuR, it suggests that the RNA-binding protein FXR1 is an IL-19 effector protein. This introduces a pathway in which IL-19-induced FXR1 expression is part of a negative compensatory, counterregulatory mechanism used by VSMCs to respond to and dampen inflammation. This mechanism is summarized in Fig. 3.
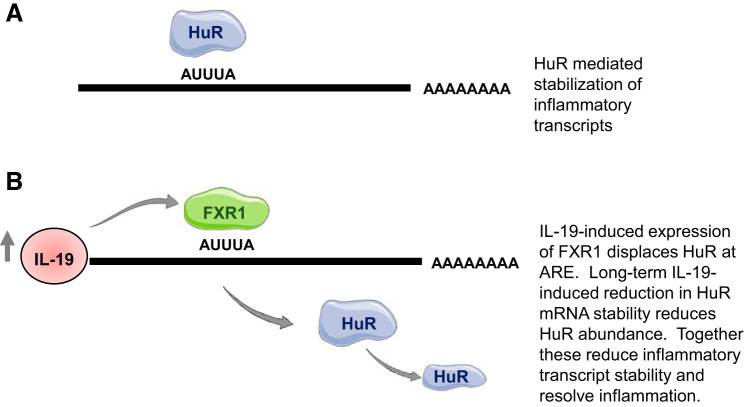
Fig. 3.
Proposed mechanism of IL-19 resolution of inflammation in vascular smooth muscle cells. A: in inflammatory situations, human R antigen (HuR) nucleocytoplasmic translocation stabilizes inflammatory transcripts containing AU-rich elements (ARE). B: in inflammatory resolution, IL-19 induces fragile X mental retardation syndrome-related protein 1 (FXR1) expression, which displaces HuR binding to ARE, reducing inflammatory mRNA stability. FXR1 also displaces HuR interaction to ARE on the HuR mRNA transcript. With long-term IL-19 stimulation, HuR abundance is reduced, further reducing inflammatory mRNA stability.
Summary and Resolution
There is an old maxim: “what goes up, must come down.” This certainly applies to inflammation, and our potent and complex inflammatory response, by necessity, needed to evolve an equally potent and complex mechanism for its resolution. The literature concerning IL-19 is complex and often seemingly contradictory, and very likely tissue and disease dependent. There are, however, some general conclusions that can be drawn from the literature. IL-19 expression is increased in response to inflammatory stimuli, but the mechanisms of IL-19 response to these stimuli, and perhaps resolution of inflammation are diverse and cell-type specific. IL-19 decreases expression of proinflammatory cytokines in some cell types, yet may increase expression of certain proinflammatory cytokines in other cell types. In immune cells, IL-19 does appear to polarize T cells and macrophages to the Th2 and M2 phenotype, respectively. In nonimmune cells, IL-19 can directly reduce stability of proinflammatory mRNA transcripts through an HuR-dependent mechanism. Considering these conclusions, one certainty is that IL-19 role in modulation of the inflammatory response is nuanced and complex, and almost undoubtedly dependent on tissue-specific receptor expression. Future studies in a variety of cell types and disease models are required to determine the precise molecular and cellular mechanisms for IL-19-mediated resolution of inflammation. Although it appears that STAT3 is activated by IL-19 in most cell types tested, what are the detailed signaling and gene expression patterns distal to IL-19 STAT3 activation, and are these pathways unique to different cell types? In the broader picture, IL-19 tissue-specific knockout animals may be necessary to better characterize IL-19 inflammation resolving effects in different disease models. For example, a keratinocyte-specific knockout mouse may distinguish if IL-19 anti-inflammatory effects are direct on keratinocytes, and not secondary from polarization of adaptive immunity and less inflammatory cross talk with those cells. Similarly, are IL-19 antiatherogenic effects due to direct inflammation-resolving activity on resident vascular cells, or secondary from immune cell polarization, or a combination of both? A second fruitful approach might be tissue-specific knockout of one of the IL-20 receptors, thus rendering a particular tissue refractory to IL-19 inflammation-dampening effects. Taken together, several investigators using a variety of approaches are likely necessary to “resolve” IL-19’s anti-inflammatory effects.
GRANTS
This work was supported by the NIH National Heart, Lung, and Blood Institute Grants HL141108 and HL117724 to M. V. Autieri and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK11042 to R. G. Scalia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.L. and M.V.A. prepared figures; T.L. and M.V.A. drafted manuscript; T.L., R.G.S., and M.V.A. edited and revised manuscript; T.L., R.G.S., and M.V.A. approved final version of manuscript.
REFERENCES
- 1.An W, Yu Y, Zhang Y, Zhang Z, Yu Y, Zhao X. Exogenous IL-19 attenuates acute ischaemic injury and improves survival in male mice with myocardial infarction. Br J Pharmacol 176: 699–710, 2019. doi: 10.1111/bph.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm Bowel Dis 16: 1017–1028, 2010. doi: 10.1002/ibd.21151. [DOI] [PubMed] [Google Scholar]
- 3.Bakheet T, Frevel M, Williams BR, Greer W, Khabar KS. ARED: human AU-rich element-containing mRNA database reveals an unexpectedly diverse functional repertoire of encoded proteins. Nucleic Acids Res 29: 246–254, 2001. doi: 10.1093/nar/29.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barreau C, Paillard L, Osborne HB. AU-rich elements and associated factors: are there unifying principles? Nucleic Acids Res 33: 7138–7150, 2005. doi: 10.1093/nar/gki1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckley CD, Gilroy DW, Serhan CN, Stockinger B, Tak PP. The resolution of inflammation. Nat Rev Immunol 13: 59–66, 2013. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Caspi RR, Chong WP. IL-20 receptor cytokines in autoimmune diseases. J Leukoc Biol 104: 953–959, 2018. doi: 10.1002/JLB.MR1117-471R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol 121: 1108–1111, 2008. doi: 10.1016/j.jaci.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Cooley ID, Chauhan VS, Donneyz MA, Marriott I. Astrocytes produce IL-19 in response to bacterial challenge and are sensitive to the immunosuppressive effects of this IL-10 family member. Glia 62: 818–828, 2014. doi: 10.1002/glia.22644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuneo AA, Herrick D, Autieri MV. IL-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol 49: 647–654, 2010. doi: 10.1016/j.yjmcc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai W, Zhang G, Makeyev EV. RNA-binding protein HuR autoregulates its expression by promoting alternative polyadenylation site usage. Nucleic Acids Res 40: 787–800, 2012. doi: 10.1093/nar/gkr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal 20: 2165–2173, 2008. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol 167: 3545–3549, 2001. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 13.Eberhardt W, Doller A, Akool S, Pfeilschifter J. Modulation of mRNA stability as a novel therapeutic approach. Pharmacol Ther 114: 56–73, 2007. doi: 10.1016/j.pharmthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ellison S, Gabunia K, Kelemen SE, England RN, Scalia R, Richards JM, Orr AW, Traylor JG Jr, Rogers T, Cornwell W, Berglund LM, Goncalves I, Gomez MF, Autieri MV. Attenuation of experimental atherosclerosis by interleukin-19. Arterioscler Thromb Vasc Biol 33: 2316–2324, 2013. doi: 10.1161/ATVBAHA.113.301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 decreases leukocyte-endothelial cell interactions by reduction in endothelial cell adhesion molecule mRNA stability. Am J Physiol Cell Physiol 305: C255–C265, 2013. doi: 10.1152/ajpcell.00069.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17: 3448–3460, 1998. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. Circ Res 118: 703–720, 2016. doi: 10.1161/CIRCRESAHA.115.306300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fredman G, Tabas I. Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am J Pathol 187: 1211–1221, 2017. doi: 10.1016/j.ajpath.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145: 33–43, 1999. doi: 10.1016/S0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 20.Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov 15: 551–567, 2016. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- 21.Gabunia K, Ellison S, Kelemen S, Kako F, Cornwell WD, Rogers TJ, Datta PK, Ouimet M, Moore KJ, Autieri MV. IL-19 halts progression of atherosclerotic plaque, polarizes, and increases cholesterol uptake and efflux in macrophages. Am J Pathol 186: 1361–1374, 2016. doi: 10.1016/j.ajpath.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabunia K, Herman AB, Ray M, Kelemen SE, England RN, DeLa Cadena R, Foster WJ, Elliott KJ, Eguchi S, Autieri MV. Induction of MiR133a expression by IL-19 targets LDLRAP1 and reduces oxLDL uptake in VSMC. J Mol Cell Cardiol 105: 38–48, 2017. doi: 10.1016/j.yjmcc.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev 21: 345–352, 2010. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, Dickensheets H, Donnelly RP. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol 4: 615–626, 2004. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Garnon J, Lachance C, Di Marco S, Hel Z, Marion D, Ruiz MC, Newkirk MM, Khandjian EW, Radzioch D. Fragile X-related protein FXR1P regulates proinflammatory cytokine tumor necrosis factor expression at the post-transcriptional level. J Biol Chem 280: 5750–5763, 2005. doi: 10.1074/jbc.M401988200. [DOI] [PubMed] [Google Scholar]
- 26.Hao S, Baltimore D. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat Immunol 10: 281–288, 2009. doi: 10.1038/ni.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman AB, Vrakas CN, Ray M, Kelemen SE, Sweredoski MJ, Moradian A, Haines DS, Autieri MV. FXR1 is an IL-19-responsive RNA-binding protein that destabilizes pro-inflammatory transcripts in vascular smooth muscle cells. Cell Rep 24: 1176–1189, 2018. doi: 10.1016/j.celrep.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev 31: 1–15, 2016. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horiuchi H, Parajuli B, Wang Y, Azuma YT, Mizuno T, Takeuchi H, Suzumura A. Interleukin-19 acts as a negative autocrine regulator of activated microglia. PLoS One 10: e0118640, 2015. doi: 10.1371/journal.pone.0118640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges. Brief Funct Genomics 12: 489–498, 2013. doi: 10.1093/bfgp/elt028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jordan WJ, Eskdale J, Boniotto M, Lennon GP, Peat J, Campbell JDM, Gallagher G. Human IL-19 regulates immunity through auto-induction of IL-19 and production of IL-10. Eur J Immunol 35: 1576–1582, 2005. doi: 10.1002/eji.200425317. [DOI] [PubMed] [Google Scholar]
- 32.Kako F, Gabunia K, Ray M, Kelemen SE, England RN, Kako B, Scalia RG, Autieri MV. Interleukin-19 induces angiogenesis in the absence of hypoxia by direct and indirect immune mechanisms. Am J Physiol Cell Physiol 310: C931–C941, 2016. doi: 10.1152/ajpcell.00006.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konrad RJ, Higgs RE, Rodgers GH, Ming W, Qian YW, Bivi N, Mack JK, Siegel RW, Nickoloff BJ. Assessment and clinical relevance of serum IL-19 levels in psoriasis and atopic dermatitis using a sensitive and specific novel immunoassay. Sci Rep 9: 5211–5253, 2019. doi: 10.1038/s41598-019-41609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 10: 387–398, 1999. doi: 10.1016/S1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 35.Kragstrup TW, Andersen T, Holm C, Schiøttz-Christensen B, Jurik AG, Hvid M, Deleuran B. Toll-like receptor 2 and 4 induced interleukin-19 dampens immune reactions and associates inversely with spondyloarthritis disease activity. Clin Exp Immunol 180: 233–242, 2015. doi: 10.1111/cei.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp Dermatol 15: 991–1004, 2006. doi: 10.1111/j.1600-0625.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 37.Lal A, Mazan-Mamczarz K, Kawai T, Yang X, Martindale JL, Gorospe M. Concurrent versus individual binding of HuR and AUF1 to common labile target mRNAs. EMBO J 23: 3092–3102, 2004. doi: 10.1038/sj.emboj.7600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, Cheng KC, Lee MF, Chiang SR, Shieh JM, Chang MS. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J Immunol 1950: 6712–6718, 2004. doi: 10.4049/jimmunol.173.11.6712. [DOI] [PubMed] [Google Scholar]
- 39.Libby P, Hansson GK. Taming immune and inflammatory responses to treat atherosclerosis. J Am Coll Cardiol 71: 173–176, 2018. doi: 10.1016/j.jacc.2017.10.081. [DOI] [PubMed] [Google Scholar]
- 40.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4+ Th2 lymphocyte function. J Immunol 1950: 3146–3155, 2001. doi: 10.4049/jimmunol.167.6.3146. [DOI] [PubMed] [Google Scholar]
- 41.Matsuo Y, Azuma YT, Kuwamura M, Kuramoto N, Nishiyama K, Yoshida N, Ikeda Y, Fujimoto Y, Nakajima H, Takeuchi T. Interleukin 19 reduces inflammation in chemically induced experimental colitis. Int Immunopharmacol 29: 468–475, 2015. doi: 10.1016/j.intimp.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA 102: 8686–8691, 2005. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R. Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol 4: 577–592, 2004. doi: 10.1016/j.intimp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Nathan C, Ding A. Nonresolving inflammation. Cell 140: 871–882, 2010. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Nikfarjam BA, Ebtekar M, Sabouni F, Pourpak Z, Kheirandish M. Detection of interleukin-19 mRNA in C57BL/6 mice astroglial cells and brain cortex. Basic Clin Neurosci 5: 88–95, 2014. [PMC free article] [PubMed] [Google Scholar]
- 46.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol 36: 380–388, 2006. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 47.Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 72: 856–863, 2002. [PubMed] [Google Scholar]
- 48.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 22: 929–979, 2004. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 49.Ray M, Gabunia K, Vrakas CN, Herman AB, Kako F, Kelemen SE, Grisanti LA, Autieri MV. Genetic deletion of IL-19 (interleukin-19) exacerbates atherogenesis in Il19−/−×Ldlr−/− double knockout mice by dysregulation of mRNA stability protein HuR (human antigen R). Arterioscler Thromb Vasc Biol 38: 1297–1308, 2018. doi: 10.1161/ATVBAHA.118.310929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards J, Gabunia K, Kelemen SE, Kako F, Choi ET, Autieri MV. Interleukin-19 increases angiogenesis in ischemic hind limbs by direct effects on both endothelial cells and macrophage polarization. J Mol Cell Cardiol 79: 21–31, 2015. doi: 10.1016/j.yjmcc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 377: 1119–1131, 2017. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 52.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326: 298–304, 1992. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 53.Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol 172: 1500–1508, 2008. doi: 10.2353/ajpath.2008.070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw G, Kamen R. A conserved AU sequence from the 3′ untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell 46: 659–667, 1986. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 55.Sun DP, Yeh CH, So E, Wang LY, Wei TS, Chang MS, Hsing CH. Interleukin (IL)-19 promoted skin wound healing by increasing fibroblast keratinocyte growth factor expression. Cytokine 62: 360–368, 2013. doi: 10.1016/j.cyto.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Förster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 10: 39–49, 1999. doi: 10.1016/S1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 57.Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol 173: 901–909, 2008. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahl C, Müller W, Leithäuser F, Adler G, Oswald F, Reimann J, Schirmbeck R, Seier A, Weiss JM, Prochnow B, Wegenka UM. IL-20 receptor 2 signaling down-regulates antigen-specific T cell responses. J Immunol 1950: 802–810, 2009. doi: 10.4049/jimmunol.182.2.802. [DOI] [PubMed] [Google Scholar]
- 59.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC Jr. Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis 146: 109–115, 1992. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 60.Wegenka UM. IL-20: biological functions mediated through two types of receptor complexes. Cytokine Growth Factor Rev 21: 353–363, 2010. doi: 10.1016/j.cytogfr.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol 172: 567–576, 2004. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 62.Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, Wittig BM, Warszawska K, Kurek A, Erdmann-Keding M, Kunz S, Asadullah K, Kadin ME, Volk HD, Sterry W, Wolk K, Sabat R. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol 134: 2757–2767, 2014. doi: 10.1038/jid.2014.308. [DOI] [PubMed] [Google Scholar]
- 63.Xie W, Fang L, Gan S, Xuan H. Interleukin-19 alleviates brain injury by anti-inflammatory effects in a mice model of focal cerebral ischemia. Brain Res 1650: 172–177, 2016. doi: 10.1016/j.brainres.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Yang J, Gao YD, Guo W. Th17 immunity in patients with allergic asthma. Int Arch Allergy Immunol 151: 297–307, 2010. doi: 10.1159/000250438. [DOI] [PubMed] [Google Scholar]