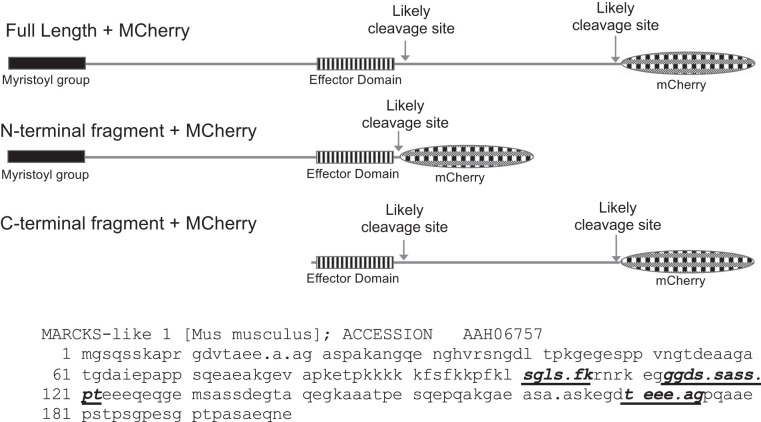
Fig. 5.
Schematic diagram of myristoylated alanine-rich protein kinase C substrate (MARCKS)-like protein-1 (MLP-1) cleavage sites. Three MLP-1 constructs: the top is a full-length construct with likely cleavage sites marked (see Table 2); the center is the NH2-terminal fragment; and the bottom is the COOH-terminal fragment. All of the fragments contain a COOH-terminal mCherry epitope and an intact effector domain. Below: MLP-1 sequence showing high-probability calpain cleavage sites as bold, italic, underlined residues. Periods mark 2 other calpain cleavage sites (Table 2) that would produce very short products near the NH2- or COOH-terminal ends of MLP-1. The initial methionine is a signal peptide that is cleaved early in protein maturation. The full-length construct is residues 2–200 + mCherry (29 kDa; NH2-terminal fragment is residues 2–118 + mCherry; COOH-terminal fragment is residues 112–200 + mCherry). Residue 131 is an alternative start site in the COOH-terminal fragment.