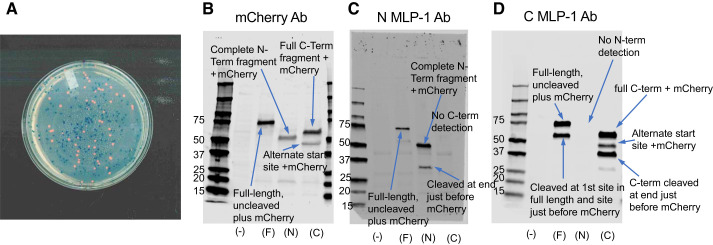
Fig. 6.
Cleavage of myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1). A: the expression of constructs that contained mCherry produced characteristically pink colonies in contrast to the blue of the colonies that contained no insert. B–D: the expression patterns we observed that were detected with an mCherry antibody (Ab) or antibodies to the NH2-terminal or COOH-terminal epitope of MLP-1 are consistent with 2 cleavage sites, 1 near the middle of the molecule and 1 just before the COOH terminus of MLP-1 that cleaves off the mCherry epitope (Fig. 5). In B–D, (–), F, N, and C are untransfected and transfected with full-length MLP + mCherry, NH2-terminal MLP + mCherry, or COOH-terminal MLP + mCherry, respectively. B: the mCherry Ab also detected the 112-residue NH2-terminal (N-Term) fragment + mCherry at ~51 kDa and the 118-residue COOH-terminal (C-Term) fragment + mCherry at 58 kDa. It also detected a 43-kDa band that is likely produced by an alternative start site corresponding to the methionine at position 131 (a 68-residue fragment + mCherry). C: the NH2-terminal antibody detected the same full-length band and NH2-terminal fragment as mCherry Ab. As expected, it detected nothing in the COOH-terminal lane. It did, however, detect a lower molecular mass band at 32 kDa in the NH2-terminal lane that was not detected by mCherry Ab. This band is consistent with full-length MLP-1 cleaved just before the mCherry tag after the effector domain (Fig. 5). D: the COOH-terminal antibody also detected the same full-length band, COOH-terminal fragment, and alternative start fragment as mCherry Ab. As expected, it detected nothing in the NH2-terminal lane. It did, however, detect a lower molecular mass band at 33 kDa in the COOH-terminal lane that was not detected by mCherry Ab. This band is consistent with the COOH-terminal fragment cleaved just before the mCherry tag (Fig. 5). Molecular mass is in kilodaltons.