Fig. 10.
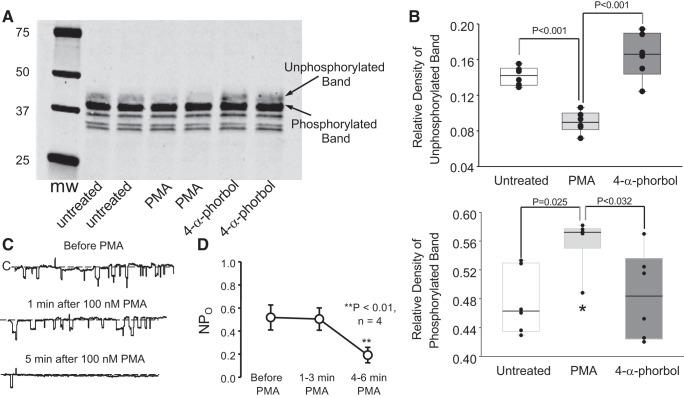
Phorbol esters increase myristoylated alanine-rich protein kinase C substrate-like protein-1 (MLP-1) phosphorylation and inhibit epithelial sodium channel (ENaC). Endogenous MLP-1 is a target for protein kinase C phosphorylation. We applied phorbol myristoyl acetate to activate protein kinase C and prepared cell lysates. Western blots (A) of the lysates showed a strong band at 37 kDa, which is characteristic of MLP-1. Just above it is the nonphosphorylated band, which is expected to migrate more slowly on the SDS gel. PMA reduces the density of the unphosphorylated band (B, top; P < 0.001, 1-way analysis of variance on ranks; n = 6) and increases the density of the phosphorylated band (B, bottom; *P < 0.025, 1-way analysis of variance on ranks; n = 6). As control (C), we also applied an inactive phorbol, which does not change the relative density of the phosphorylated and nonphosphorylated bands. We also used single-channel methods (see methods) to examine principal cells in isolated, split-open collecting ducts bathed in the same saline that we used to obtain A and B. As expected, addition of PMA rapidly reduced ENaC channel activity, defined as the number of channels within the patch (N) times the channel open probability (Po) (C and D). **P < 0.01, Kruskal–Wallis 1-way analysis of variance on ranks; 4 patches on 4 principal cells from 4 mice of any sex; n = 4. mw, Molecular weight (i.e., molecular mass, in kilodaltons).