Abstract
miRNAs provide fine tuning of gene expression via inhibition of translation. miR-451 has a modulatory role in cell cycling via downregulation of mechanistic target of rapamycin. We aimed to test whether chronic systemic inhibition of miR-451 would enhance renal fibrosis (associated with deranged autophagy). Adult TallyHo/Jng mice (obese insulin resistant) were randomized to two treatment groups to receive either miR-451 inhibition [via a locked nucleic acid construct] or a similar scrambled locked nucleic acid control for 8 wk. All mice were fed a high-fat diet (60% kcal from fat) ad libitum and humanely euthanized after 12 wk. Kidneys and blood were collected for analysis. Renal expression of miR-451 was sixfold lower in inhibitor-treated mice compared with control mice. miR-451 inhibition increased kidney weight and collagen and glycogen deposition. Blood chemistry revealed significantly higher Na+ and anion gap (relative metabolic acidosis) in inhibitor-treated mice. Western blot analysis and immunohistochemistry of the kidney revealed that the inhibitor increased markers of renal injury and fibrosis, e.g., kidney injury molecule 1, neutrophil gelatinase-associated lipocalin, transforming growth factor-β, 14-3-3 protein-ζ, mechanistic target of rapamycin, AMP-activated protein kinase-α, calcium-binding protein 39, matrix metallopeptidase-9, and the autophagy receptor sequestosome 1. In contrast, the inhibitor reduced the epithelial cell integrity marker collagen type IV and the autophagy markers microtubule-associated protein 1A/1B light chain 3B and beclin-1. Taken together, these results support a protective role for miR-451 in reducing renal fibrosis by enhancing autophagy in obese mice.
Keywords: autophagy, diabetes, diabetic nephropathy, microRNAs
INTRODUCTION
Diabetic nephropathy (DN) is the most common and severe diabetic complication and is the leading cause of end-stage renal disease (31). Damage to the kidney is initiated by poor glycemic control and elevated blood pressure transmitted to the kidney (poor autoregulation of blood pressure) (41, 49), resulting in ultrastructural, morphological, and functional changes (2, 39, 41). This progresses to microalbuminuria and then macroalbuminuria (DN), glomerular basement membrane thickening, Kimmelsteil-Wilson nodules, tubular hypertrophy, and accumulation of the extracellular matrix in the kidney (2, 39, 41).
Understanding the mechanisms governing the development and progression of DN is complicated by the plethora of signaling pathways activated, generating several pathological substances (7), including advanced glycation end products, transforming growth factor-β (TGF-β), and reactive oxygen species (22, 24, 48). Although there has been significant progress made in our understanding of protein changes in DN, finer aspects of regulatory control have yet to be elucidated.
miRNAs constitute one such layer of additional fine tuning of gene expression that has recently achieved greater appreciation. miRNAs, which number over 1,000 distinct species, constitute small (18–25 nt) endogenous noncoding RNAs. They have been demonstrated to be involved in nearly all central cellular homeostatic processes, including cell proliferation, cell differentiation, and cell death (12). Dysregulated and altered miRNA expression has been implicated in the pathogenesis of both nondiabetic and diabetic kidney disease (14, 16, 21, 23, 26, 28, 33, 45, 49, 55). Early evidence that miRNAs are involved in the development of DN was provided from studies of mice with podocyte-specific deletion of Dicer, an important cytoplasmic ribonuclease in the processing of mature miRNAs (18). Additional investigations revealed a variety of kidney-enriched miRNAs and regional expression patterns, many of which modulate fibrotic pathways (39, 43, 45).
miRNAs are sorted into classes based on predicted targets. We have developed an interest in miR-451, since we found it increased nearly 1,000-fold in the urine exosomes of streptozotocin-treated type 1 diabetic rats (34). Surprisingly, in that study, increased severity of diabetes in the rats was negatively correlated with kidney cortical levels of miR-451, suggesting that miR-451 may have a protective role. An early study showed that miR-451 regulates terminal erythropoiesis (40). However, more recent work suggests the ability of miR-451 to regulate components of inflammatory and fibrotic pathways in the kidney (15, 29, 44, 52). miR-451 was found downregulated in the kidneys of db/db mice (leptin receptor mutation), contributing to mesangial hypertrophy (44, 52). Moreover, miR-451 administered via vector was shown to inhibit glomerular mesangial growth via downregulation of tyrosine 3-monooxygenase/tryptophan 5-monooygenase activation protein-ζ (YWHAZ or 14-3-3 protein-ζ) and p38 MAPK signaling (52) in the db/db mouse. Collectively, these findings suggest that miR-451 may have protective actions in reducing the severity of DN, ultimately promising unique interventions.
In addition to its role in p38 MAPK signaling in the kidney, YWHAZ may attenuate apoptosis and/or autophagy (38, 52). Autophagy is a highly conserved process necessary for maintaining cellular homeostasis by eliminating cytotoxic protein aggregates and damaged organelles through lysosomal degradation (6). Impairment of the autophagy process has been associated with the development and progression of DN. YWHAZ, of which there are several isoforms, regulates phosphorylative signaling via strategic binding (inhibition of nuclear translocation). This class of proteins (activated by growth factors and MAPK pathways) has been determined to have a role in the promotion of oncogenic and chemoresistant pathways (37). Similar pathways are known to be overactivated in DN.
In the present study, we investigated the hypothesis that miR-451 confers protection against DN by targeting key components of the autophagy process in the diabetic kidney using locked nucleic acid (LNA) technology. LNAs are modified oligos with unparalleled sensitivity and specificity compared with traditional DNA or RNA oligos (9). In addition, LNAs provide increased potency both in vivo and in vitro (9, 11, 35). More recently, this sophisticated technology has promised to provide new and exciting methods to advance our understanding of disease progression and miR-based therapeutics, including DN (8, 39). We tested our hypothesis in vivo in TallyHo/Jng (TH) mice. TH mice have several quantitative trait loci linked to hyperglycemia, insulin resistance, type 2 diabetes, and adiposity (13). We fed mice a high-fat diet (HFD) and administered a LNA antagonist specific to miR-451 to monitor its effects on disease progression in DN. We also confirmed several direct effects of the LNA constructs in cell culture using human proximal tubule (PT) cells.
METHODS
Ethics statement/study approval.
All animal experiments were approved (protocol 2016-1358) by the Georgetown University Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal protocol.
Insulin-resistant male TH mice (strain no. 005314, The Jackson Laboratory, Bar Harbor, ME) bred at Georgetown University were studied between the ages of 4 and 7 mo. For miR-451 expression using the in situ hybridization approach, we also studied eight male C57Bl6 mice maintained on normal chow diet. TH mice were placed on a HFD (60% kcal from fat, Research Diets). Only male mice were used for these experiments, since female mice develop much less severe obesity and insulin resistance. After 1 wk on HFD, mice were randomized into two groups (n = 8 mice/group) and received an intraperitoneal injection of either LNA-miR-451a-inhibitor or scrambled LNA control (scramble) one time weekly (both at 2 mg/kg) for 8 consecutive weeks. Lyophilized LNA inhibitor probe (product name: mmu-miR-451a inh, product sequence: 5′-G*T*A*A*T*G*G*T*A*A*C*G*G*T*T-3′) and LNA-scramble (product name: negative ctrl A, product sequence: 5′-A*C*G*T*C*T*A*T*A*C*G*C*C*A-3′) were purchased from Exiqon (now sold by Qiagen, Hilden, Germany) and resuspended in sterile Tris-EDTA buffer (pH 8). Fasting (6 h) and nonfasting blood glucose were measured from the tail vein using a glucometer (OneTouch, Lifescan, Malvern, PA). Urine (24 h) was collected in mouse metabolic cages (Hatteras Instruments) at baseline and 2, 4, 6, and 8 wk after the first injection. At 12.5 wk, mice were euthanized, and kidneys and blood were collected under Inactin (thiobutabarbital) anesthesia (80 mg/kg·body wt ip in ~200 μL PBS). A consort diagram of the study flow is provided in Supplemental Fig. S1 in the Supplemental Material (https://doi.org/10.6084/m9.figshare.11923134.v2).
Blood and urine analysis.
Blood collected via cardiac puncture was analyzed using an iSTAT portable analyzer (Abbott). Urine (24 h) was analyzed for albumin (Albuwell M; Exocell) and creatinine (Cayman Chemical) by ELISA and colorimetric assays, respectively.
Kidney tissue histology and immunohistochemistry.
During euthanasia, the right kidney was perfusion fixed, and the left kidney was rapidly frozen for protein and RNA analyses. Sectioned kidneys were stained with Masson’s trichrome (MT) and periodic acid-Schiff (PAS) at the Histopathology and Tissue Shared Resources Center (Georgetown University). MT-stained sections were qualitatively analyzed for the degree of collagen (blue stain) using an Olympus BX43 microscope. Glomeruli from PAS-stained sections were similarly analyzed for the degree of glycogen deposition. Immunoperoxidase-based immunohistochemistry for YWHAZ and mechanistic target of rapamycin (mTOR) was performed on sectioned kidneys as previously described (46) using commercial antibodies YWHAZ (14-3-3-ζ, 1:200, rabbit monoclonal, D7H5, Cell Signaling) and mTOR (1:200, rabbit monoclonal, 7C10, Cell Signaling).
In situ hybridization.
We performed in situ hybridization on sections prepared by the Histopathology and Tissue Shared Resources Center using RNase-free techniques and LNA detection probes (Qiagen). Hybridization was performed with a kit (BioChain, Newark, CA) following the procedures outlined by the manufacturer.
Quantitative real-time PCR.
Total RNA was extracted from whole kidneys using a miRNeasy Mini Kit (Qiagen). Reverse transcription was performed using an miScript II RT Kit (Qiagen). Quantitative RT-PCR was performed in duplicate using an miScript SYBR Green PCR Kit and miScript Primer Assay for miR-451a (catalog no. MS00002408, Qiagen). Amplification followed by a melting curve step to confirm the specificity of amplification was carried out in a 96-well plate using the Applied Biosystems OneStep-Plus. All procedures were performed following instructions outlined by the manufacturer. Data were normalized using RNU6 as an endogenous control (catalog no. MS00033740, Qiagen). Changes in miRNA gene expression were calculated using the ΔΔCt method (where Ct is threshold cycle) and with commercial software provided by the manufacturer.
Western blot analysis.
Western blot analysis was performed on cortex homogenates dissected from the left kidney and homogenized as previously described (13). Briefly, cortex homogenates were solubilized in Laemmli sample buffer, and 10–20 μg of protein were loaded in precast gels (Criterion, TGX Bio-Rad, Hercules, CA). Separated proteins were transferred to nitrocellulose membrane and blocked with 5% nonfat milk for 1 h. Membranes were then incubated with their respective primary antibodies. Antibodies used included: AMP-actived protein kinase-α (AMPKα; rabbit monoclonal, D63G4, Cell Signaling), MO25α/calcium-binding protein 39 (CAB39; rabbit monoclonal, C49D8, Cell Signaling), TGF-β (rabbit monoclonal, no. 3711, Cell Signaling), YWHAZ, 14-3-3ζ (rabbit monoclonal, D7H5, Cell Signaling), mTOR (rabbit monoclonal, 7C10, Cell Signaling), microtubule-associated protein 1A/1B light chain 3B [LC3(A/B); rabbit monoclonal, no. 4108, Cell Signaling], matrix metallopeptidase-9 (MMP-9; rabbit polyclonal, PA5-13199, ThermoFisher), glucose-6-phosphatase catalytic subunit (G6PC; rabbit polyclonal, PA5-42541, ThermoFisher), collagen type IV (Col IV; rabbit polyclonal, ab6586, Abcam), kidney injury molecule 1 (KIM-1; rabbit polyclonal, ab47635, Abcam), neutrophil gelatinase-associated lipocalin (NGAL; rabbit polyclonal, ab63929, Abcam), p70 S6 kinase 1 (S6K1; rabbit polyclonal, ab9366, Abcam), sequestosome 1 (SQSTM1/p62; rabbit polyclonal, ab155686, Abcam), beclin-1 (rabbit monoclonal, ab207612, Abcam), and LC3-I/II (rabbit polyclonal, ABC929, Sigma-Aldrich). Protein loading was normalized by Ponceau-stained membranes before probing with primary antibodies. Blots were developed either to film or with an Amersham 600 digitized imaging device.
Cell culture experiments.
For primary cultures, kidney tissue samples were obtained from three different human kidneys from patients undergoing radical nephrectomy for renal cell carcinoma at the Department of Urology at the Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India. The experiments were conducted after approval from the Institutional Ethics Committee (IEC code 2014-182-IP-81) and consent from the subjects. Tissue samples (~10 mg) were collected from areas microscopically identified as normal (in the cortex) immediately after the specimen extraction by an expert uropathologist. Specimens were minced into small pieces and transferred to a sterile 50-mL tube with collagenase solution [Dulbecco’s PBS with 0.1% (wt/vol) collagenase type 2]. Samples were incubated for 30 min at 37°C with gentle stirring. After digestion, tissue was passed onto the first sieve (100 mm) followed by a 70-mm sieve and subsequently washed two times by centrifugation at 1,500 g for 5 min at 4°C. Next, pellets were resuspended in DMEM-F-12 culture medium with 12% FBS. The isolated cells were seeded on collagen-coated culture flasks and left unstirred for 48 h at 37°C in a humidified incubator (95% air-5% CO2). Thereafter, the medium was replaced every 2 days. Typically, after 7 days, cells were found to be organized as a confluent monolayer. Cells between the second and fourth passage were used for the experiments. For immunofluorescence, cells were characterized by positive staining for aquaporin 1 (AQP1) protein. Human PT cells were grown on coverslips. They were fixed and permeabilized with 100% methanol followed by incubation with 3% BSA in PBS plus Tween 20 for 1 h to block nonspecific protein-protein interactions. Cells were then incubated with primary antibody against AQP1 (ab168387) at a dilution of 1:200 overnight at 4°C. The secondary antibody used was Alexa Fluor 488-labeled goat anti-rabbit IgG (H+L) (ab150077) at a dilution of 1:500 for 1 h at room temperature. DAPI was used to stain cell nuclei at a concentration of 1 µg/mL. Slides were mounted, covered, and air dried. Cells were viewed and photographed with a Nikon UV fluorescence microscope. Images were taken at ×10 magnification.
For RNA interference experiments, 60–70% confluent cells were transfected with miRCURY LNA, a miRNA inhibitor against human miR-451a [Homo sapiens (HSA-)miR-451a inhibitor, 50 nM), and miRCURY LNA negative, a miRNA inhibitor control (HSA-miR-451a negative control, 50 nM) as per the manufacturer’s instructions (Qiagen). Lipofectamine RNAiMAX transfection reagent (Invitrogen) was used for transfection (36). After 48 h of transfection, cells were harvested for total RNA extraction and RT-PCR analysis using commercially available probes for miR-451 (TaqMan MicroRNA Assay, assay ID 001141, ThermoFisher), CAB39 (forward: 5′-CACGTTTTTAAGGTGTTTGTAGCC-3′ and reverse: 5′-ATCCTCCGTCCTGTCGTTCTG-3′), and YWHAZ (forward: 5′-TGATCCCCAATGCTTCACAAG-3′ and reverse: 5′-GCCAAGTAACGGTAGTAATCTCC-3′).
Statistical analyses.
Group sizes were set to n = 8 a priori using power analysis with power set at 0.85, α set at 0.05, and an effect size of 20%, using pilot SD of Western blot data (13%). The resulting data are, in general, presented as means ± SE. Outliers were excluded using the 1.5 interquartile rule. Data were analyzed by an unpaired Student’s t test using GraphPad Prism version 7. P < 0.05 was considered statistically significant between the two groups.
RESULTS
Physiological parameters and blood chemistry.
Body weight, heart weight, and final blood glucose were not significantly different between miR-451 inhibitor and scramble-treated mice, although there was a trend for body weight-normalized heart weight to be greater in inhibitor-treated mice (P = 0.064; Table 1). Blood chemistry revealed a small but significant elevation of blood Na+ and anion gap in inhibitor-treated mice. In contrast, blood urea nitrogen was significantly reduced. Overall, urine volumes decreased over time in both treatment groups, potentially related to the HFD (see Supplemental Fig. S2, available online at https://doi.org/10.6084/m9.figshare.11923134.v2). There were trends for reductions in blood bicarbonate and pH in the inhibitor-treated mice.
Table 1.
Physiological parameters in control and inhibitor-treated Tally-Ho/Jng mice
| Control | Inhibitor | P Value | |
|---|---|---|---|
| Body weight, g | 48.1 ± 1.7 | 43.9 ± 2.2 | 0.16 |
| Heart weight, g | 0.201 ± 0.006 | 0.208 ± 0.008 | 0.53 |
| Heart weight, g/40 g body wt | 0.168 ± 0.004 | 0.192 ± 0.012 | 0.064 |
| Glucose, mmol/L | 14.4 ± 2.1 | 13.0 ± 1.3 | 0.60 |
| Na+, mmol/L | 144 ± 1 | 147 ± 1 | 0.007* |
| Urine volume, ml/24 h† | 0.44 ± 0.07 | 0.50 ± 0.08 | 0.57 |
| K+, mmol/L | 4.86 ± 0.19 | 4.89 ± 0.14 | 0.92 |
| Cl−, mmol/L | 118 ± 1 | 118 ± 1 | 0.95 |
| Blood urea nitrogen, mg/dL | 22.1 ± 0.9 | 18.6 ± 0.6 | 0.006* |
| BEecf, mmol/L | 3.75 ± 1.93 | −1.25 ± 1.51 | 0.06 |
| Hct, %PCV | 29.3 ± 1.6 | 30.6 ± 1.09 | 0.48 |
| , mmol/L | 30.6 ± 1.5 | 26.7 ± 1.2 | 0.07 |
| AnGap, mmol/L | 0.75 ± 1.93 | 8.00 ± 1.77 | 0.015* |
| Hb, g/dL | 9.94 ± 0.53 | 10.4 ± 0.4 | 0.47 |
| pH, −log[H+] | 7.27 ± 0.03 | 7.21 ± 0.02 | 0.09 |
Data are means ± SE; n = 8 mice/treatment. BEecf, base excess in extracellular fluid; Hct, hematocrit; PCV, packed cell volume; AnGap, anion gap; Hb, hemoglobin.
Significantly different as determined by an unpaired t test.
Final collection.
miR-451 inhibition reduces renal expression of miR-451.
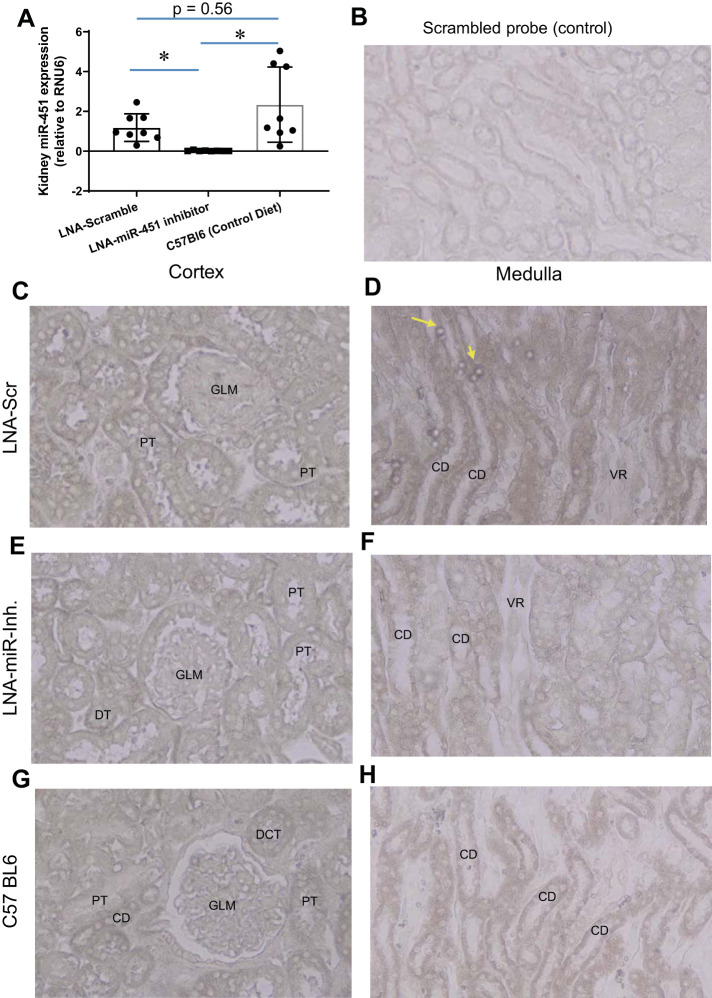
To get a better sense of how renal miR-451 expression in obese TH mice fed a HFD compared with a more common strain of conventionally fed mice, we conducted expression analysis of the kidney in three groups, including C57Bl6 male mice fed a normal diet (Fig. 1A). Only inhibitor-treated mice showed significantly reduced miR-451. Expression of miR-451 (by in situ hybridization) appeared to be greatest overall in distal segments of the kidney (distal convoluted tubule, and collecting duct; Fig. 1, C and D) in scramble-treated mice and reduced overall in inhibitor-treated mice (Fig. 1, E and F). Figure 1, G and H, shows miR-451 labeling in the kidney of C57Bl6 male mice, which appeared slightly less intense (in tubules) than that observed in the scramble-treated kidney.
Fig. 1.
Systemic inhibition of miR-451 reduces renal miR-451 expression. A: kidney whole homogenate expression of miR-451 (data normalized to RNU6) in scrambled locked nucleic acid (LNA; scramble)-treated TallyHo/Jng (TH), LNA-miR-451 inhibitor-treated TH, and untreated C57Bl6 male mice. In situ hybridization using a nonspecific probe (B) and using a specific miR-451 probe (C–H) is shown. C and D: cortex (C) and medulla (D) from an LNA scramble-treated TH mouse. E and F: cortex (E) and medulla (F) from an LNA-miR-451 inhibitor-treated TH mouse. G and H: cortex (G) and medulla (H) from an untreated C57Bl6 mouse (×400). Yellow arrows point to erythrocytes that have high levels of miR-451 in scramble-treated mice. CD, collecting duct; PT, proximal tubule; VR, vasa recta; GLM, glomerulus; DT, distal tubule; DCT, distal convoluted tubule. *Tukey’s multiple-comparison test was used to determine significance, with P < 0.05 considered significant (n = 8 mice/group).
Kidney weight is increased by miR-451 inhibition.
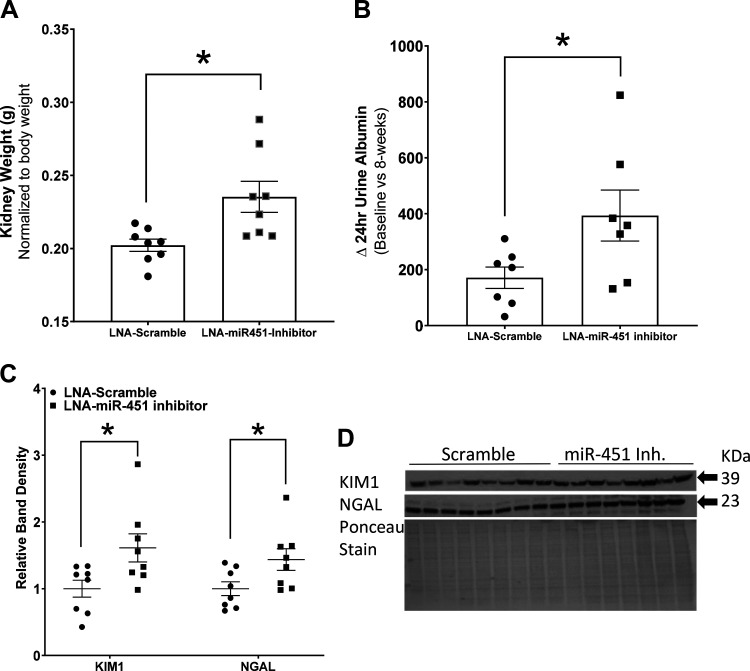
Body weight-normalized kidney weight was increased in inhibitor-treated mice (Fig. 2A). Inhibitor-treated mice also had a significantly greater rise in 24-h urine albumin compared with scramble-treated control mice (Fig. 2B). Densitometric analysis of Western blots for markers of kidney injury, using whole cortex homogenates, revealed significant increases in both NGAL and KIM-1 in inhibitor-treated mice (Fig. 2, C and D).
Fig. 2.
Increased markers of renal injury in mice with systemic miR-451 inhibition. A: kidney weight (normalized to body weight). B: change (Δ) in 24-h urine albumin at baseline versus 8 wk. C: relative density summaries for kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL). D: representative Western blots and Ponceau stain (normalizing) for KIM-1 and NGAL. LNA, locked nucleic acid. *An unpaired t test was used to determine significance, with P < 0.05 considered significant (n = 7–8 mice/group).
Histological evidence of renal injury.
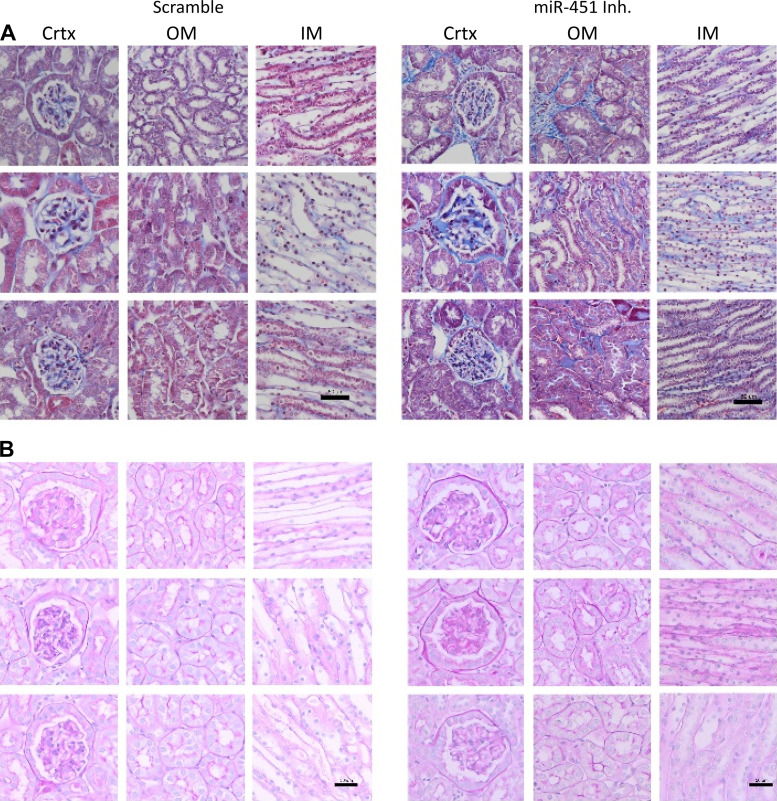
Collagen deposition (based on MT staining) appeared more prevalent in the inner medulla, cortex, and outer medulla in inhibitor-treated mice relative to scramble-treated control mice (Fig. 3A). Similarly, analysis of PAS-stained images of glomeruli suggested more glycogen deposition in the mesangium of inhibitor-treated mice (Fig. 3, C and D).
Fig. 3.
Inhibition of miR-451 increases collagen and glomerular glycogen deposition in TallyHo/Jng (TH) mice. A: representative Masson’s trichrome staining in the cortex (Crtx), outer medulla (OM), and inner medulla (IM) from treated mice. Scale bar = 50 µm. B: representative periodic acid-Schiff staining in the cortex, outer medulla, and inner medulla from treated mice. Scale bars = 50 µm.
miR-451 inhibition increases renal fibrotic signaling.
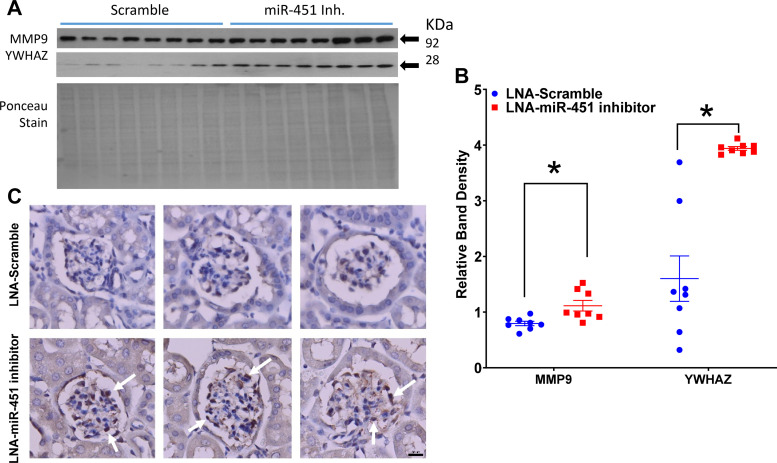
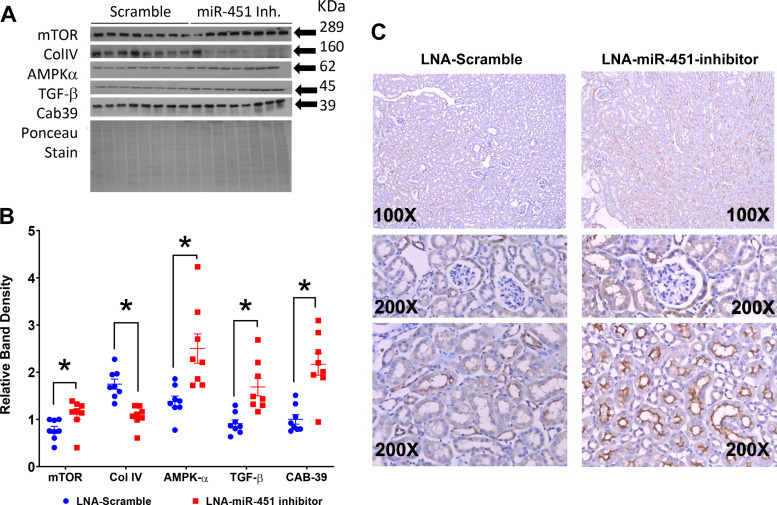
Systemic inhibition of miR-451 led to an increase in the predicted targets of miR-451, i.e., MMP-9 and YWHAZ (Fig. 4, A and B). More specifically, immunohistochemistry revealed an increase in YWHAZ in the mesangium of inhibitor-treated mice (Fig. 4, C and D). mTOR, AMPKα, and TGF-β were increased significantly in the inhibitor-treated mice, whereas Col IV was decreased in this group (Fig. 5, A and B). Immunohistochemistry of mTOR revealed largely apical staining in tubules, especially in the medulla (Fig. 5C).
Fig. 4.
Systemic inhibition of miR-451 increases predicted targets of miR-451 in the cortex of TallyHo/Jng (TH) mice. A and B: Western blot analysis (A) and relative density summaries (B) for tyrosine 3-monooxygenase/tryptophan 5-monooygenase activation protein-ζ (YWHAZ) and matrix metallopeptidase-9 (MMP-9) (normalized to Ponceau red stain). C: representative YWHAZ staining showing the increase in YWHAZ protein in glomeruli [mesangium (white arrows)] of inhibitor-treated mice. Scale bar = 50 µm. LNA, locked nucleic acid. Data are presented as means ± SE. *An unpaired t test was used to determine significance, with P < 0.05 considered significant.
Fig. 5.
Systemic inhibition of miR-451 increases fibrotic and mechanistic target of rapamycin (mTOR) signaling in the cortex. A and B: Western blot analysis (A) and relative (normalized to Ponceau stain) density summaries (B) for mTOR, collagen type IV (Col IV), AMP-activated protein kinase-α (AMPKα), transforming growth factor-β (TGF-β), and calcium-binding protein 39 (CAB39). C: representative mTOR immunohistochemistry (IHC) in scramble- and inhibitor-treated mice. LNA, locked nucleic acid. Data are presented as means ± SE; n = 8 mice/group. An unpaired t test was used to determine significance, with P < 0.05 considered significant. *P < 0.05.
miR-451 inhibition led to expression changes supporting reduced autophagy.
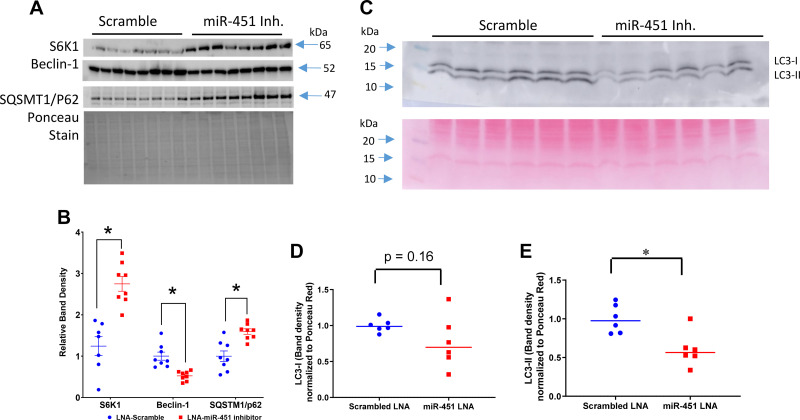
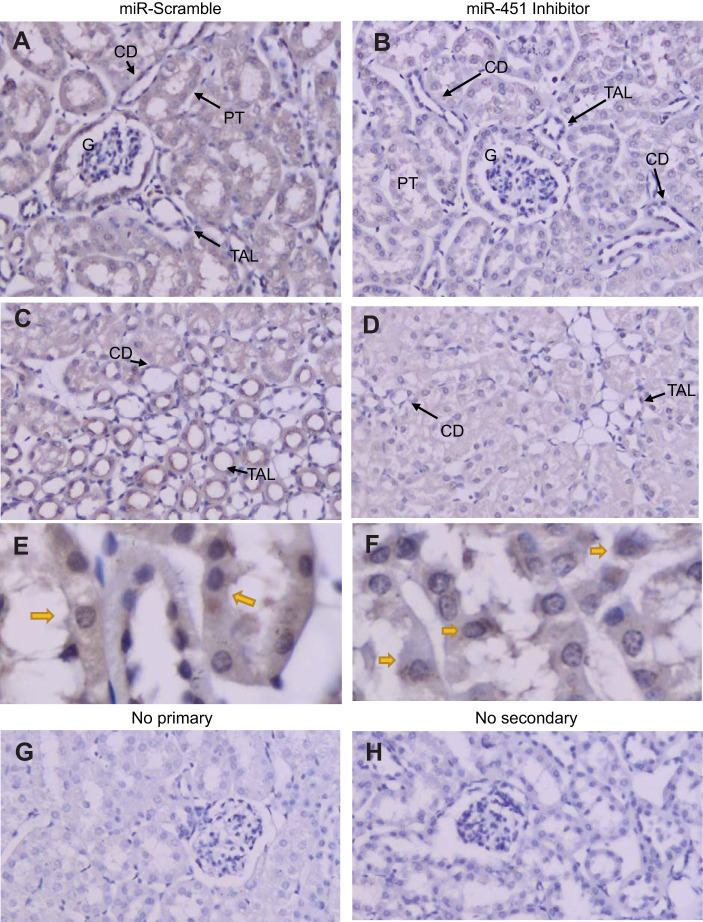
Autophagy markers, e.g., SQSTM1/p62, beclin-1, LC3, and S6K1, were evaluated by Western blot analysis in cortex homogenates (Fig. 6). S6K1 (mTOR substrate) and p62 (a signaling adapter degraded primarily by autophagy) were significantly higher in inhibitor-treated mice. Conversely, beclin-1 (a core component of the phosphoinositide 3-kinase complex involved in stimulating autophagy) was significantly lower in inhibitor-treated mice (Fig. 6, A and B). The conversion of LC3-I to LC3-II due to a phosphatidylethanolamine conjugation allows it to be recruited to autophagosomal membranes. LC3-II band density was significantly reduced in miR-451 inhibitor-treated mice (Fig. 6, C–E). There was also a strong trend for LC3-I to be reduced (P = 0.16). LC3-I/II cellular localization and abundance were also assessed by immunohistochemistry (Fig. 7). LC3-I/II staining was diffuse in PTs and more apical in the thick ascending limb (two tubular sites of the most distinct signal). The intensity of the stain was greater in scramble-treated mice. There was also greater evidence of punctate staining in scramble-treated mice, an indication of LC3-II in the autophagosome (Fig. 7E).
Fig. 6.
Assessment of cortex autophagy process in TallyHo/Jng (TH) mice. A and B: Western blot analysis (A) and relative band density summaries (B; normalized to Ponceau stain) for p70 S6 kinase 1 (S6K1), beclin-1, and sequestosome 1 (SQSTM1/p62). C–E: representative Western blot analysis (C) and density summaries for microtubule-associated protein 1A/1B light chain 3B (LC3)-I (D) and LC3-II (E). LNA, locked nucleic acid. Data are presented as means ± SE; n = 6–8 mice/group. *An unpaired t test was used to determine significance, with P < 0.05 considered significant.
Fig. 7.
Immunohistochemistry for microtubule-associated protein 1A/1B light chain 3B (LC3)-I/II in TallyHo/Jng (TH) mice. A and B: kidney cortex of a locked nucleic acid scramble-treated TH mouse (A) and an inhibitor-treated TH (B) mouse probed with antibody against LC3-I/II (×200). C and D: medullary sections for scramble-treated (C) and inhibitor-treated (D) mice. E and F: higher magnifications (×600, oil immersion) of proximal tubule (PT) in scramble-treated (E) and inhibitor-treated (F) mice. Yellow arrows indicate apparently greater membrane/cytosolic versus nuclear staining in scramble-treated mice. G and H: tissue processed for immunohistochemistry (IHC) with no primary (G) or secondary (H) antibodies. CD, collecting duct; TAL, thick ascending limb; G, glomerulus.
Cell culture confirms YWHAZ and CAB39 are direct targets of miR-451 in the human renal PT.
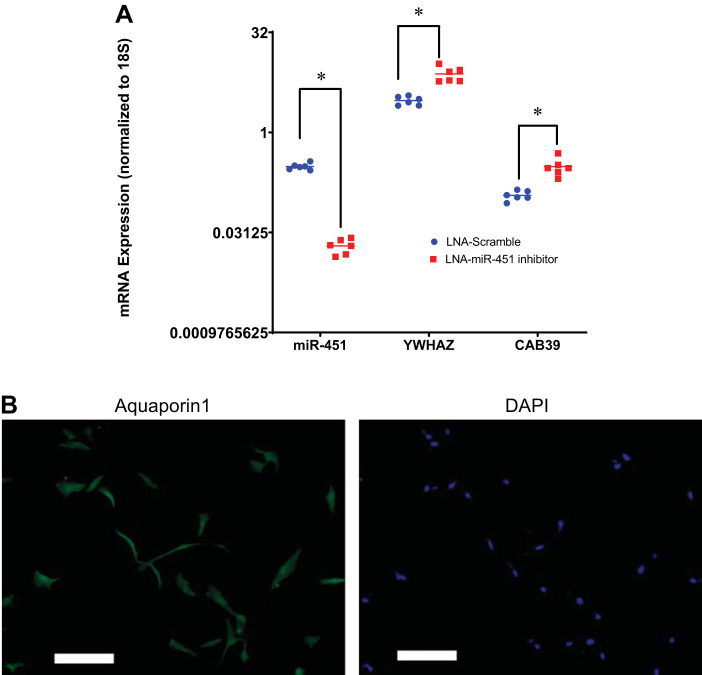
Because systemic miR-451 inhibition may have a number of indirect effects, we confirmed our hypothetical signaling pathway using human PT cells (Fig. 8). We found that LNA-miR-451 inhibition (using a construct designed against human miR-451) reduced mRNA expression of miR-451 and upregulated both YWHAZ and CAB39, validating these particular changes as likely due to direct effects of the antagonist.
Fig. 8.
Validation of direct targets of miR-451 in human proximal tubule (hPT) cells. hPT cells were brought to confluence and then treated with locked nucleic acid (LNA) miR-451a inhibitor or negative control at 50 nM. After 48 h, transfected cells were harvested, total RNA was prepared, and quantitative RT-PCR was conducted. A: fold expression (; where CT is threshold cycle) of miR-451, tyrosine 3-monooxygenase/tryptophan 5-monooygenase activation protein-ζ (YWHAZ), and calcium-binding protein 39 (CAB39) in hPT cells transfected with either Homo sapiens (hsa-)LNA-miR-451a-inhibitor or hsa-LNA-miR-451a inhibitor control (LNA scramble). Transcript levels of genes of interest were normalized against 18S rRNA. *Significant difference due to an unpaired t test (P < 0.05). B: representative immunofluorescence image of hPT cells stained for aquaporin-1 protein (green) and counterstained with DAPI (blue, nuclear stain). White lines represent scale bars of 50 μm. Images were taken at ×100 magnification.
DISCUSSION
Autophagy is a highly regulated intracellular lysosomal degradation process that functions to remove cytotoxic protein aggregates and recycle energy to maintain intracellular homeostasis (4, 6). Autophagy plays a critical role in the kidney; moreover, how miRNAs fine tune this process is an emerging area of study. It is well recognized that dysregulation of autophagy is involved in the progression of DN (5). Based on our previous work (34) and reports in the literature (17, 42, 44, 47, 53, 56), we hypothesized that miR-451 may be protective of the kidney during chronic periods of hyperglycemia (or frank type 2 diabetes) by facilitating autophagy. In support of this, we found that prolonged systemic miR-451 antagonism by a LNA construct increased kidney hypertrophy, albuminuria, and collagen and glycogen deposition in the kidneys of male TH mice. Although TH mice are an insulin-resistant mouse model, they are somewhat resistant to the development of renal inflammation, injury, and fibrosis (1). Thus, we felt this was a good model to study initiation of DN, which we predicted would be enhanced in inhibitor-treated mice.
Our antagonism scheme was able to significantly reduce renal miR-451 expression so that the mean expression level was 1.7% of the scrambled level. In agreement with previous work (predicting most likely direct targets of miR-451 based on overlapping appearances in miRanda, PicTar, and TargetScan databases; see Ref. 52), we found miR-451 antagonism resulted in upregulation of renal YWHAZ (14-3-3ζ) and CAB39. We went on to define a parallel relationship between the upregulation of these defined targets of miR-451, mTOR, inflammation, reduced autophagy, and several indexes of renal disease. Thus, we propose that miR-451 is protective of the kidney in states of high insulin signaling (hyperglycemia) via enhancement of autophagy.
The 14-3-3 proteins, including YWHAZ, comprise a large class of acidic proteins expressed within all eukaryotic cells, which regulate primarily by interacting with specific phosphoserine and phosphothreonine motifs on target kinases. YWHAZ, specifically, can retard autophagy through interaction and regulation of autophagy-promoting kinases, phosphatases, and transmembrane receptors (20). This interaction is permissible by phosphorylation of the binding partner on a serine residue. For example, phosphorylation of AKT1S1 [subunit of mTOR complex 1 (mTORC1)], a known binding partner of YWHAZ, enhances mTORC1 signaling and reduces autophagy (20). Similarly, YWHAZ binds and inhibits the action of tuberous sclerosis complex 2, upstream of mTORC1, a tumor suppressor gene and enhancer of autophagy (19). In our present study, we found that inhibition of miR-451 led to increased renal expression of mTOR and its substrate (S6K1) in TH mice, suggesting increased mTOR activity. In addition to inhibition of autophagy, overactivation of mTORC1 has been reported to stimulate the synthesis of inflammatory proteins, e.g., TGF-β, resulting in collagen fiber deposition (3, 5, 32). In support of this, we found increased TGF-β and collagen deposition in the kidneys of inhibitor-treated mice.
Our findings with regard to autophagy markers (p62, beclin-1, and LC3) were consistent in that the sequestosome (p62) was increased in inhibitor-treated mice, whereas LC3 and beclin (a core component of the autophagosome) were decreased. When autophagy signals are activated, LC3 is processed by a series of autophagy-related proteins (Atg) first to LC3-I, which has a glycine residue in the carboxy tail. Next, LC3-I is further processed by conjugation to phosphoethanolamine to produce LC3-II, which, although larger, runs faster on polyacrylamide gels than LC3-I due to hydrophobicity. LC3-II is associated with mature autophagosomes (6, 51). Although LC3-I is found in the cytoplasm, LC3-II is the active form localized to the inner and outer membrane of the autophagosome and reflects the number of autophagosomes and autophagy-related structures (6, 51). Our finding of a reduction in both LC3-I and LC3-II (even though the prior did not quite meet significance) would suggest the reduction in autophagic responses induced by miR-451 inhibition was upstream of LC3-I production rather than a deficiency in processing of LC3 by Atg proteins. Beclin-1 is another target of YWHAZ after phosphorylation by protein kinase B (Akt). This process enhances the formation of the beclin-1/YWHAZ/vimentin complex, which inhibits autophagy in renal injury, as well as promotes tumorigenesis in cancer (25). An impaired autophagy response in these mice is further supported by the increased expression of p62, the signaling adapter degraded by autophagy, in mice treated with miR-451 inhibitor.
In addition to YWHAZ-mediated regulation of mTOR, miR-451 may also regulate mTOR via CAB39 (53). CAB39, another validated target of miR-451, regulates responses to metabolic stress. In glioma cells, Zhao et al. (53) demonstrated that upregulation of miR-451 suppressed the activation of the AMPK/mTOR pathway via downregulation of CAB39, whereas downregulation of miR-451 activated the same pathway via increased CAB39. Similar findings were recently reported in erythrocytes (a rich source of miR-451), demonstrating a role for miR-451 in activation of the AMPK/mTOR signaling cascade via CAB39 (10). We found that inhibition of miR-451 resulted in increased CAB39 in both mouse native kidneys and human PT cells. Linkage of the AMPK/mTOR pathway to renal autophagy has already been established in acute kidney injury (54). Our study extends these findings to a role for this pathway in DN and modifiable by miR-451.
Another predicted target of miR-451 is MMP-9, which we found substantially increased with inhibition of miR-451. MMP-9 is a known collagenase, specifically digesting Col IV in the basement membrane (30). In agreement, we found reduced Col IV by immunoblot analysis in the cortex of inhibitor-treated mice. In our recent collaborative effort, we found that levels of MMP-9 were increased in diabetic kidney tissue in rats (34). In addition, Li et al. (30) evaluated the role of MMP-9 in DN in vivo and in vitro and found that deficiency of MMP-9 attenuates diabetic kidney injury. Taken together, these findings suggest MMP-9 is detrimental to renal epithelial integrity.
Thus, we envision early renal injury in type 2 diabetes is at least partially initiated by overactivity of receptor tyrosine kinase signaling (insulin), which activates AKT and mTORC1. miR-451 would serve then to suppress mTORC1 growth-related signaling to protect against both tumors as well as excessive collagen, matrix, and glycogen deposition.
It is important to point out that in our study miR-451 antagonism (given systemically) would not only affect the kidney but any number of other organs/systems. In fact, a study by Kuwabara et al. (27) demonstrated a role for miR-451 in exacerbating cardiac hypertrophy in high fat-fed C57Bl6 mice. In their study, cardiac-specific deletion of miR-451 was protective of this hypertrophy. Further mechanistic examination suggested that the cardiac cells were stressed by high levels of dietary palmitic acid, leading to miR-451 expression, CAB39 repression, and increased cell toxicity. We did not observe treatment differences in heart weight in our study, suggesting no impact of the inhibition of miR-451 on high dietary-fat-induced cardiac hypertrophy.
Finally, miR-451 expression has been shown to be reduced in the kidney in severe DN (44, 50), which we consider to be “uncompensated” DN. We believe, under persistent diabetic conditions, that miR-451 regulation is lost and its expression is drastically reduced, resulting in the upregulation of YWHAZ (14-3-3ζ) signaling and protein binding. In turn, this may lead to hyperactivation of mTOR, resulting in enhanced downstream effects like protein synthesis, cellular proliferation, and an impaired autophagy response in the kidneys, exacerbating the renal injury phenotype. Overall, this study suggests that miR-451 offers protection against renal fibrosis by regulating autophagy in the kidney. Furthermore, our present findings provide foundational support for additional studies on the role of miR-451 in DN and the potential of miRNA-based therapeutics to ameliorate disease progression.
GRANTS
The research reported in this publication was supported by National Center for Advancing Translational Sciences Grant TL1TR001431 (to M. B. Fluitt) and American Diabetes Association Award 1-18-PMF-002 (to M. B. Fluitt). This work was additionally supported in part by the Georgetown University Medical Center’s Faculty Research Support, Department of Medicine, Georgetown University (to C. M. Ecelbarger). Cell culture experiments were partially supported by a grant (to S. Tiwari) from the Indian Council of Medical Research, Government of India [Coord/7 (1)/CARE-KD/2018/NCD-II, no. 5/4/7-12/13/NCD-II].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T. and C.M.E. conceived and designed research; M.B.F., M.K., S.S., L.L., and C.M.E. performed experiments; M.B.F., N.S., S.T., and C.M.E. interpreted results of experiments; M.B.F., N.S., S.T., and C.M.E. analyzed data; M.B.F. and C.M.E. prepared figures; M.B.F. and C.M.E. drafted manuscript; M.B.F., N.S., M.K., L.L., S.T., and C.M.E. edited and revised manuscript; M.B.F., N.S., M.K., S.S., L.L., S.T., and C.M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Daniel A. Ecelbarger (script for Masson’s trichrome pixel density) and Dr. Alexandra R. Piselli (Western blot analysis) for technical support.
REFERENCES
- 1.Betz B, Conway BR. An update on the use of animal models in diabetic nephropathy research. Curr Diab Rep 16: 18, 2016. doi: 10.1007/s11892-015-0706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol 791: 8–24, 2016. doi: 10.1016/j.ejphar.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 3.Brown AL, Fluitt MB, Ecelbarger CM. Mechanistic target of rapamycin: integrating growth factor and nutrient signaling in the collecting duct. Am J Physiol Renal Physiol 315: F413–F416, 2018. doi: 10.1152/ajprenal.00170.2018. [DOI] [PubMed] [Google Scholar]
- 4.Cai J, Zhang H, Zhang YF, Zhou Z, Wu S. MicroRNA-29 enhances autophagy and cleanses exogenous mutant αB-crystallin in retinal pigment epithelial cells. Exp Cell Res 374: 231–248, 2019. doi: 10.1016/j.yexcr.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Choi ME. Autophagy in diabetic nephropathy. J Endocrinol 224: R15–R30, 2015. doi: 10.1530/JOE-14-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebrahim N, Ahmed IA, Hussien NI, Dessouky AA, Farid AS, Elshazly AM, Mostafa O, Gazzar WBE, Sorour SM, Seleem Y, Hussein AM, Sabry D. Mesenchymal stem cell-derived exosomes ameliorated diabetic nephropathy by autophagy induction through the mTOR signaling pathway. Cells 7: 226, 2018. doi: 10.3390/cells7120226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 30: 49–59, 2012. doi: 10.1111/j.1755-5922.2010.00218.x. [DOI] [PubMed] [Google Scholar]
- 8.Elmén J, Lindow M, Silahtaroglu A, Bak M, Christensen M, Lind-Thomsen A, Hedtjärn M, Hansen JB, Hansen HF, Straarup EM, McCullagh K, Kearney P, Kauppinen S. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucleic Acids Res 36: 1153–1162, 2008. doi: 10.1093/nar/gkm1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elmén J, Thonberg H, Ljungberg K, Frieden M, Westergaard M, Xu Y, Wahren B, Liang Z, Ørum H, Koch T, Wahlestedt C. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res 33: 439–447, 2005. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang X, Shen F, Lechauve C, Xu P, Zhao G, Itkow J, Wu F, Hou Y, Wu X, Yu L, Xiu H, Wang M, Zhang R, Wang F, Zhang Y, Wang D, Weiss MJ, Yu D. miR-144/451 represses the LKB1/AMPK/mTOR pathway to promote red cell precursor survival during recovery from acute anemia. Haematologica 103: 406–416, 2018. doi: 10.3324/haematol.2017.177394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fluiter K, ten Asbroek AL, de Wissel MB, Jakobs ME, Wissenbach M, Olsson H, Olsen O, Oerum H, Baas F. In vivo tumor growth inhibition and biodistribution studies of locked nucleic acid (LNA) antisense oligonucleotides. Nucleic Acids Res 31: 953–962, 2003. doi: 10.1093/nar/gkg185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fluitt MB, Kumari N, Nunlee-Bland G, Nekhai S, Gambhir KK. miRNA-15a, miRNA-15b, and miRNA-499 are reduced in erythrocytes of pre-diabetic African-American adults. Jacobs J Diabetes Endocrinol 2: 014, 2016. [PMC free article] [PubMed] [Google Scholar]
- 13.Fluitt MB, Rizvi S, Li L, Alunan A, Lee H, Tiwari S, Ecelbarger CM. chronic insulin infusion down-regulates circulating and urinary nitric oxide (NO) levels despite molecular changes in the kidney predicting greater endothelial NO synthase activity in mice. Int J Mol Sci 19: 2880, 2018. doi: 10.3390/ijms19102880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francoz C, Nadim MK, Durand F. Kidney biomarkers in cirrhosis. J Hepatol 65: 809–824, 2016. doi: 10.1016/j.jhep.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Gits CM, van Kuijk PF, Jonkers MB, Boersma AW, Smid M, van Ijcken WF, Coindre JM, Chibon F, Verhoef C, Mathijssen RH, den Bakker MA, Verweij J, Sleijfer S, Wiemer EA. MicroRNA expression profiles distinguish liposarcoma subtypes and implicate miR-145 and miR-451 as tumor suppressors. Int J Cancer 135: 348–361, 2014. doi: 10.1002/ijc.28694. [DOI] [PubMed] [Google Scholar]
- 16.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10: 593–601, 2008. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 17.Guo R, Gu J, Zhang Z, Wang Y, Gu C. MiR-451 promotes cell proliferation and metastasis in pancreatic cancer through targeting CAB39. BioMed Res Int 2017: 2381482, 2017. doi: 10.1155/2017/2381482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Harvey SJ, Jarad G, Cunningham J, Goldberg S, Schermer B, Harfe BD, McManus MT, Benzing T, Miner JH. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol 19: 2150–2158, 2008. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoki K. Role of TSC-mTOR pathway in diabetic nephropathy. Diabetes Res Clin Pract 82, Suppl 1: S59–S62, 2008. doi: 10.1016/j.diabres.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Jia H, Liang Z, Zhang X, Wang J, Xu W, Qian H. 14-3-3 proteins: an important regulator of autophagy in diseases. Am J Transl Res 9: 4738–4746, 2017. [PMC free article] [PubMed] [Google Scholar]
- 21.Kantharidis P, Wang B, Carew RM, Lan HY. Diabetes complications: the microRNA perspective. Diabetes 60: 1832–1837, 2011. doi: 10.2337/db11-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 6: 395–423, 2011. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol 4: 1255–1266, 2009. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato M, Natarajan R. MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets. Ann N Y Acad Sci 1353: 72–88, 2015. doi: 10.1111/nyas.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kidd ME, Shumaker DK, Ridge KM. The role of vimentin intermediate filaments in the progression of lung cancer. Am J Respir Cell Mol Biol 50: 1–6, 2014. doi: 10.1165/rcmb.2013-0314TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kort EJ, Farber L, Tretiakova M, Petillo D, Furge KA, Yang XJ, Cornelius A, Teh BT. The E2F3-Oncomir-1 axis is activated in Wilms’ tumor. Cancer Res 68: 4034–4038, 2008. doi: 10.1158/0008-5472.CAN-08-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuwabara Y, Horie T, Baba O, Watanabe S, Nishiga M, Usami S, Izuhara M, Nakao T, Nishino T, Otsu K, Kita T, Kimura T, Ono K. MicroRNA-451 exacerbates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy in mice through suppression of the LKB1/AMPK pathway. Circ Res 116: 279–288, 2015. doi: 10.1161/CIRCRESAHA.116.304707. [DOI] [PubMed] [Google Scholar]
- 28.Lee SO, Masyuk T, Splinter P, Banales JM, Masyuk A, Stroope A, Larusso N. MicroRNA15a modulates expression of the cell-cycle regulator Cdc25A and affects hepatic cystogenesis in a rat model of polycystic kidney disease. J Clin Invest 118: 3714–3724, 2008. doi: 10.1172/JCI34922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li HY, Zhang Y, Cai JH, Bian HL. MicroRNA-451 inhibits growth of human colorectal carcinoma cells via downregulation of Pi3k/Akt pathway. Asian Pac J Cancer Prev 14: 3631–3634, 2013. doi: 10.7314/APJCP.2013.14.6.3631. [DOI] [PubMed] [Google Scholar]
- 30.Li SY, Huang PH, Yang AH, Tarng DC, Yang WC, Lin CC, Chen JW, Schmid-Schönbein G, Lin SJ. Matrix metalloproteinase-9 deficiency attenuates diabetic nephropathy by modulation of podocyte functions and dedifferentiation. Kidney Int 86: 358–369, 2014. doi: 10.1038/ki.2014.67. [DOI] [PubMed] [Google Scholar]
- 31.Liu F, Zhang ZP, Xin GD, Guo LH, Jiang Q, Wang ZX. miR-192 prevents renal tubulointerstitial fibrosis in diabetic nephropathy by targeting Egr1. Eur Rev Med Pharmacol Sci 22: 4252–4260, 2018. doi: 10.26355/eurrev_201807_15420. [DOI] [PubMed] [Google Scholar]
- 32.Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyó JM. Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17: 1395–1404, 2006. doi: 10.1681/ASN.2005050549. [DOI] [PubMed] [Google Scholar]
- 33.Lodygin D, Tarasov V, Epanchintsev A, Berking C, Knyazeva T, Körner H, Knyazev P, Diebold J, Hermeking H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 7: 2591–2600, 2008. doi: 10.4161/cc.7.16.6533. [DOI] [PubMed] [Google Scholar]
- 34.Mohan A, Singh RS, Kumari M, Garg D, Upadhyay A, Ecelbarger CM, Tripathy S, Tiwari S. Urinary exosomal microRNA-451-5p is a potential early biomarker of diabetic nephropathy in rats. PLoS One 11: e0154055, 2016. doi: 10.1371/journal.pone.0154055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mook OR, Baas F, de Wissel MB, Fluiter K. Evaluation of locked nucleic acid-modified small interfering RNA in vitro and in vivo. Mol Cancer Ther 6: 833–843, 2007. doi: 10.1158/1535-7163.MCT-06-0195. [DOI] [PubMed] [Google Scholar]
- 36.Pandey G, Shankar K, Makhija E, Gaikwad A, Ecelbarger C, Mandhani A, Srivastava A, Tiwari S. Reduced insulin receptor expression enhances proximal tubule gluconeogenesis. J Cell Biochem 118: 276–285, 2017. doi: 10.1002/jcb.25632. [DOI] [PubMed] [Google Scholar]
- 37.Pennington KL, Chan TY, Torres MP, Andersen JL. The dynamic and stress-adaptive signaling hub of 14-3-3: emerging mechanisms of regulation and context-dependent protein-protein interactions. Oncogene 37: 5587–5604, 2018. doi: 10.1038/s41388-018-0348-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pozuelo-Rubio M. 14-3-3 Proteins are regulators of autophagy. Cells 1: 754–773, 2012. doi: 10.3390/cells1040754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R. Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23: 458–469, 2012. doi: 10.1681/ASN.2011050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen KD, Simmini S, Abreu-Goodger C, Bartonicek N, Di Giacomo M, Bilbao-Cortes D, Horos R, Von Lindern M, Enright AJ, O’Carroll D. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med 207: 1351–1358, 2010. doi: 10.1084/jem.20100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson K, Wonnacott A, Fraser DJ, Bowen T. MicroRNAs in diabetic nephropathy: from biomarkers to therapy. Curr Diab Rep 16: 35, 2016. doi: 10.1007/s11892-016-0724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song L, Su M, Wang S, Zou Y, Wang X, Wang Y, Cui H, Zhao P, Hui R, Wang J. MiR-451 is decreased in hypertrophic cardiomyopathy and regulates autophagy by targeting TSC1. J Cell Mol Med 18: 2266–2274, 2014. doi: 10.1111/jcmm.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun Y, Koo S, White N, Peralta E, Esau C, Dean NM, Perera RJ. Development of a micro-array to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res 32: e188, 2004. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Peng R, Peng H, Liu H, Wen L, Wu T, Yi H, Li A, Zhang Z. miR-451 suppresses the NF-kappaB-mediated proinflammatory molecules expression through inhibiting LMP7 in diabetic nephropathy. Mol Cell Endocrinol 433: 75–86, 2016. doi: 10.1016/j.mce.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M. MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Res 18: 404–411, 2008. doi: 10.1101/gr.6587008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiwari S, Singh RS, Li L, Tsukerman S, Godbole M, Pandey G, Ecelbarger CM. Deletion of the insulin receptor in the proximal tubule promotes hyperglycemia. J Am Soc Nephrol 24: 1209–1214, 2013. doi: 10.1681/ASN.2012060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya S, Oku M, Imanaka Y, Kunimoto R, Okuno Y, Terasawa K, Sato F, Tsujimoto G, Shimizu K. MicroRNA-338-3p and microRNA-451 contribute to the formation of basolateral polarity in epithelial cells. Nucleic Acids Res 37: 3821–3827, 2009. doi: 10.1093/nar/gkp255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varghese RT, Jialal I. Diabetic Nephropathy. Treasure Island, FL: StatPearls, 2018. [Google Scholar]
- 49.Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM, Cooper ME, Kantharidis P. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes 59: 1794–1802, 2010. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H, Kong L, Zhou S, Cui W, Xu F, Luo M, Li X, Tan Y, Miao L. The role of microRNAs in diabetic nephropathy. J Diabetes Res 2014: 920134, 2014. doi: 10.1155/2014/920134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshii SR, Mizushima N. Monitoring and measuring autophagy. Int J Mol Sci 18: 1865, 2017. doi: 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Luo X, Ding S, Chen J, Chen T, Chen X, Zha H, Yao L, He X, Peng H. MicroRNA-451 regulates p38 MAPK signaling by targeting of Ywhaz and suppresses the mesangial hypertrophy in early diabetic nephropathy. FEBS Lett 586: 20–26, 2012. doi: 10.1016/j.febslet.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 53.Zhao K, Wang L, Li T, Zhu M, Zhang C, Chen L, Zhao P, Zhou H, Yu S, Yang X. The role of miR-451 in the switching between proliferation and migration in malignant glioma cells: AMPK signaling, mTOR modulation and Rac1 activation required. Int J Oncol 50: 1989–1999, 2017. doi: 10.3892/ijo.2017.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao W, Zhang L, Chen R, Lu H, Sui M, Zhu Y, Zeng L. SIRT3 protects against acute kidney injury via AMPK/mTOR-regulated autophagy. Front Physiol 9: 1526, 2018. doi: 10.3389/fphys.2018.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X, Qu Z, Zhu C, Lin Z, Huo Y, Wang X, Wang J, Li B. Identification of urinary microRNA biomarkers for detection of gentamicin-induced acute kidney injury in rats. Regul Toxicol Pharmacol 78: 78–84, 2016. doi: 10.1016/j.yrtph.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Zhuo S, Yang M, Zhao Y, Chen X, Zhang F, Li N, Yao P, Zhu T, Mei H, Wang S, Li Y, Chen S, Le Y. MicroRNA-451 negatively regulates hepatic glucose production and glucose homeostasis by targeting glycerol kinase-mediated gluconeogenesis. Diabetes 65: 3276–3288, 2016. doi: 10.2337/db16-0166. [DOI] [PubMed] [Google Scholar]