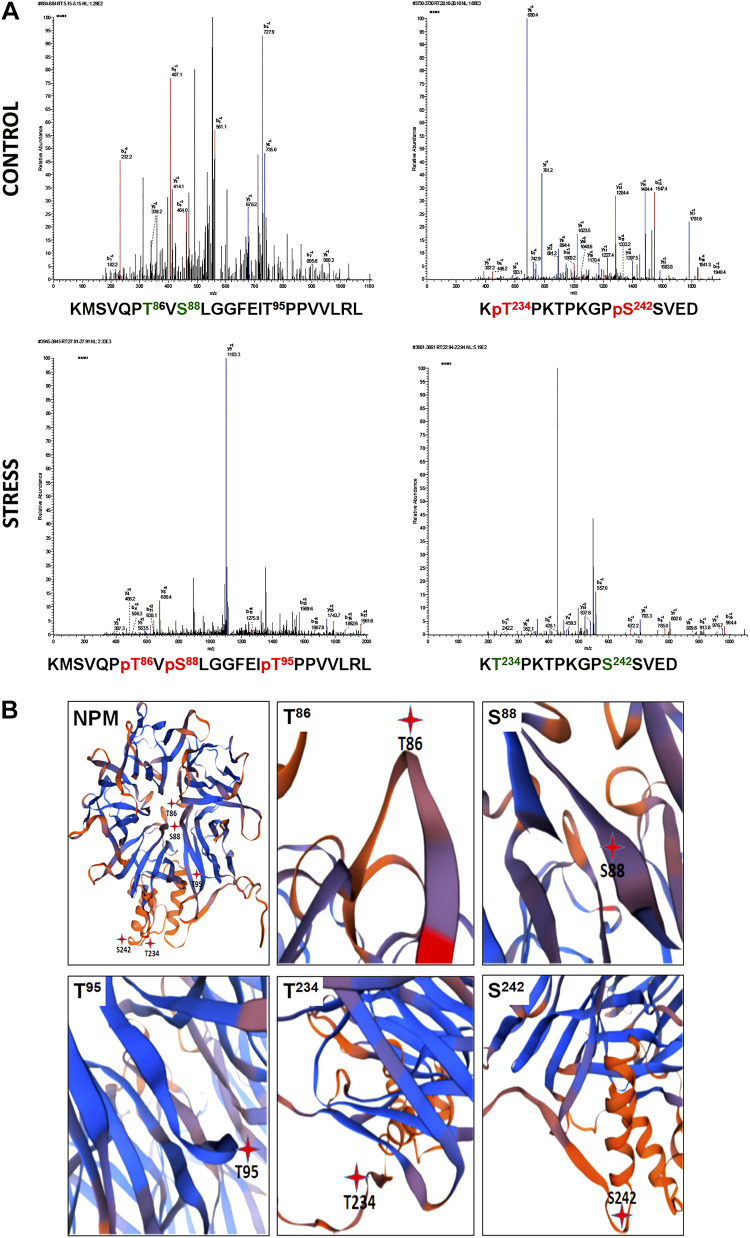
Fig. 1.
Stress causes differential nucleophosmin (NPM) phosphorylation change at NPM hinge regions. A: mass spectrometry of NPM purified from renal cortical homogenates of two kidneys from a single donor rejected for transplantation; one kidney was well perfused (control), whereas the other kidney appeared ischemic because of overt perfusion pump failure (stress). The unique peptide sequences shown below each tracing represent the specific residues that are either phosphorylated [T234 and S242 (red)] or dephosphorylated [T86, S88, and T95 (green)] during renal ischemia. MAC, mass x; MSMS, mass spectrometry/mass spectrometry. MS tracings were each repeated three times. B: differential NPM phosphorylation sites localize to hinge regions. Three-dimensional ribbon modeling showed that T86, T95, T234, and S242 residues differentially phosphorylated during stress localize to NPM hinge regions and were therefore more likely to regulate NPM conformation.