Abstract
Objective
This study aimed to evaluate the pharmacological mechanisms of antiviral drugs against the novel coronavirus disease (COVID-19) and the study designs in clinical trials registered with the International Clinical Trials Registry Platform (ICTRP).
Methods
Clinical trials involving antiviral drugs for treating COVID-19 were retrieved from the ICTRP database. For each trial, the study design, number of participants, primary endpoints, source register, antiviral mechanism, and results were evaluated.
Results
On June 10, 2020, 145 eligible clinical trials were retrieved from the ICTRP, of which 99 (68.3%) were randomized trials, 109 (75.2%) were parallel assignment trials, 38 (26.2%) were double or single blinded, 130 (89.7%) involved two groups, and 75 (51.6%) included more than 100 participants; and clinical improvement or recovery and virus-negative conversion were the two most common endpoints, accounting for 40.7% and 18.6%, respectively. The drugs were divided according to the antiviral mechanism into HIV reverse transcriptase inhibitors, RNA-dependent RNA polymerase inhibitors, HIV protease inhibitors (PIs), hepatitis C virus NS3 PIs, and anti-influenza drugs.
Conclusion
The design characteristics of clinical trials of antiviral drugs for treating COVID-19 as well as the mechanism of action and antiviral efficacy of the drugs were evaluated in this study. The results of these trials could constitute a reference for future clinical trials to be executed on COVID-19 treatment and prevention.
Keywords: COVID-19, clinical trials as topic, antiviral agents, research design
Introduction
The World Health Organization (WHO) declared on January 31, 2020, that the pathogenic viral infection currently designated as coronavirus disease (COVID-19) is a Public Health Emergency of International Concern, which has since become a pandemic; this infection is considerably transmittable and is engendered by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 The WHO’s situation report-142 reports a cumulative global total of 7,145,539 confirmed COVID-19 cases as well as 408,025 deaths as of June 10, 2020.2 In general, coronaviruses are RNA viruses belonging to the Coronaviridae family.3 Although cough and fever constitute the most frequently observed symptoms of COVID-19,4 the disease can also lead to multiple organ dysfunction, including disseminated intravascular coagulation, thromboembolism, liver and kidney injury, respiratory and circulatory failure, and even death.5 No effective antiviral drugs for COVID-19 treatment are available thus far, and thus, symptomatic support and standard care remain the main treatment methods. Nevertheless, Wu et al6 reviewed some antiviral agents (including remdesivir, favipiravir, oseltamivir, lopinavir, umifenovir, chloroquine, and hydroxychloroquine) with supporting agents (including nitric oxide, ascorbic acid, azithromycin, interleukin 6 antagonists, and corticosteroids). However, the need for more clinical trials on broad-spectrum antiviral drugs as well as antimalarial drugs for COVID-19 is urgent, particularly because the development of COVID-19 vaccines is in highly preliminary stages.
The WHO established in 2005 the International Clinical Trials Registry Platform (ICTRP) with the objective of in order to ensure that clinical trial registries are linked to provide relevant personnel scholars worldwide with an integrated portal through which they can access information on clinical trials conducted worldwide. By 2020, the ICTRP has included data from 17 national and regional clinical trial registries (https://www.who.int/en/). Accordingly, the WHO has facilitated the cultivation of the notion that controlled trials are for the global public good; thus, the ICTRP can aid health information producers as well as other related individuals to use and produce health improvement knowledge.7 The ICTRP was thus established with the aim of ensuring that all health care decision-makers are provided with a comprehensive view of research, which can consequently engender enhanced research transparency and increase validity and value of scientific evidence.
Because the development cycle of new drugs is long and the process of vaccine development is uncertain, selecting effective therapeutic agents from existing drugs is urgently needed for COVID-19 treatment. Using the registered clinical trial data on ChiCTR and ClinicalTrials.gov, we reviewed the essential attributes of China’s registered clinical trials on COVID-19.8 Antiviral and antimalarial drugs are the two main classes of therapeutic drugs that have been determined to be effective against COVID-19, but given the pandemic spread of COVID-19, the focus of most registered trials has been shifted to antiviral drugs. Consequently, the period from January to June 2020 has witnessed a considerable increase in the number of registered COVID-19 clinical trials worldwide.
Antiviral drugs designed for specific viral targets remain the most effective resources for controlling the spread of the virus. Here, we analyzed ICTRP-registered clinical trials for antiviral drugs against COVID-19 and summarized their study design and antiviral mechanism data as well as their results so as to provide a reference for COVID-19 treatment.
Materials and Methods
Data Source
We used the ICTRP, in which clinical trial data from 17 national and regional clinical trial registries are merged, as the data source.
Data Acquisition Strategy
On June 10, 2020, we searched the ICTRP for clinical trials by using the following keywords: “COVID-19” and “interventional clinical trials.” Next, we downloaded all retrieved records, and for each of them, we collated their trial identifier, public title, date registration, source register, recruitment status, target size, primary sponsor, country, study type, study design, and intervention. Moreover, two authors (ZWL, YCH) manually screened the resulting records for eligibility. Discrepancies in record classification were cleared up through discussion between the authors.
Data Extraction
Descriptive information pertaining to the following items was extracted manually from the records in the source registry by two investigators: recruitment status, target size, primary sponsor, countries, study type, study design, and intervention. For the analysis of the drug types, intervention sources were categorized. The aforementioned data were independently judged and extracted by the aforementioned authors (ZWL and HYC). Finally, a standard database was created using MS Excel for analysis.
Statistical Analyses
We herein present categorical variables and continuous ones as numbers (percentages) and medians (interquartile ranges [IQRs]), respectively. SAS (version 9.4; SAS Institute Inc., Cary, NC, USA) constituted the platform on which we executed all statistical analyses.
Results
Number of Eligible Clinical Trials
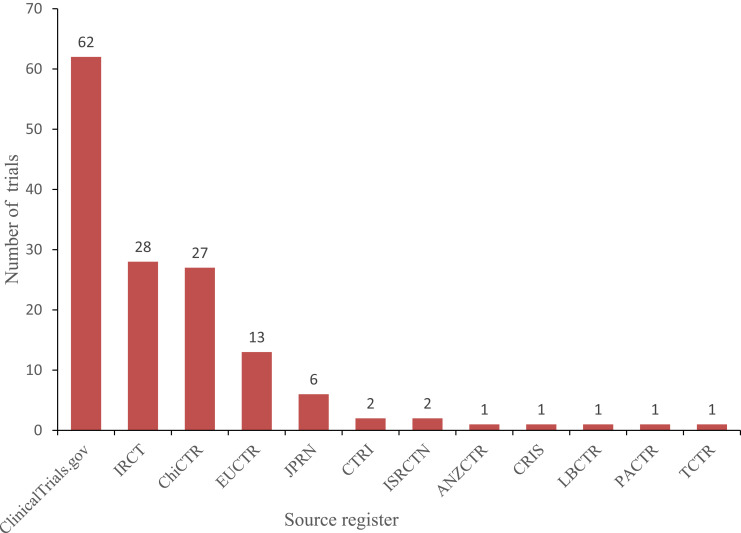
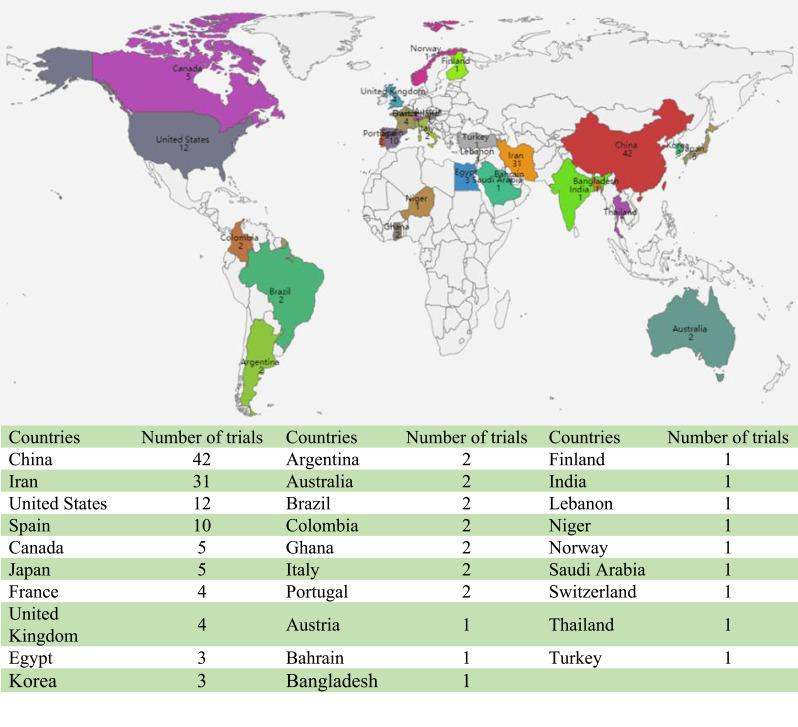
As of June 10, 2020, 1969 interventional clinical trials were registered in the ICTRP. Of these, 145 were defined as clinical trials for antiviral drugs and could be classified by their specific registries as follows: ClinicalTrials.gov (62, 42.8%), IRCT (28, 19.3%), ChiCTR (27, 18.6%), EU Clinical Trials Register (13, 9.0%), JPRN (6, 4.1%), CTRI (2, 1.4%), ISRCTN (2, 1.4%), ANZCTR (1, 0.7%), CRIS (1, 0.7%), LBCTR (1, 0.7%), PACTR (1, 0.7%), TCTR (1, 0.7%; Figure 1). By country of registration, the top 10 countries conducting these trials were as follows: China (42, 29.0%), Iran (26, 9.3%), the United States (12, 8.3%), Spain (10, 6.9%), Canada (5, 3.4%), Japan (5, 3.4%), France (4, 2.8%), the United Kingdom (4, 2.8%), Egypt (3, 2.1%), and South Korea (3, 2.1%; Figure 2).
Figure 1.
Source registries of the 145 included clinical trials for COVID-19. Copyright © 2020. Dove Medical Press. Adapted from Huang J, He Y, Su Q, et al. Characteristics of COVID-19 Clinical Trials in China Based on the Registration Data on ChiCTR and ClinicalTrials.gov. Drug Des Devel Ther. 2020; 14:2159-2164.8
Figure 2.
Countries of the 145 included clinical trials for COVID-19.
General Analysis of the Included Clinical Trials
The clinical trials could be categorized as recruiting (76, 52.4%), not recruiting (56, 38.6%), and authorized (13, 9.0%) according to the recruitment status; as randomized (99, 68.3%), nonrandomized (12, 8.3%), and not provided (34, 23.6%) according to the type of allocation; as parallel assignment (109, 75.2%), single-group assignment (12, 8.3%), nonparallel assignment (6, 4.1%), factorial assignment (3, 2.1%), sequential assignment (2, 1.4%), adaptive assignment (2, 1.4%), and not provided (11, 7.6%) according to the intervention model; and double-blinded (25, 17.2%), single-blinded (13, 9.0%), nonblinded (64, 44.1%), and not provided (43, 29.7%) according to blinding type. The numbers and proportions of clinical trials are listed as follows by phase: Phase 0 (13, 9.0%), Phase I or Phase I/II (3, 2.1%), Phase II (20, 13.8%), Phase II/III (19, 13.1%), Phase III (58, 40.0%), and Phase IV (16, 11.0%). The remainder (16, 11.0%) of the studies did not provide phase information. Moreover, 15 trials involved a single group (10.3%), 81 involved two groups (55.9%), 23 involved three groups (15.9%), 13 involved four groups (9.0%), and 13 involved five or more groups (9.0%).
In all 145 trials, the sample sized ranged between 4 and 100,000 individuals, with a cumulative statistical sample size of 512,975 cases. Moreover, a minimum sample size of 4 individuals was used in the trial NCT04324489 executed by the Renmin Hospital of Wuhan University; this trial was terminated early because the outbreak was brought under control in China. Moreover, the largest sample size of 100,000 was used in four clinical trials, namely EUCTR2020-001366-11-ES, EUCTR2020-001366-11-IT, EUCTR2020-001366-11-IE, and EUCTR2020-001366-11-LT; all these trials involved administration of the local standard care plus one antiviral agent (selected from a group of four options) and were registered in Spain and Portugal. The median (IQR) number of participants per trial and per group was 108 (50–510) and 50 (26–210), respectively. In summary, 48.3%, 26.9%, 6.9%, 10.3%, 4.1%, and 3.4% of the trials included ≤100, 101–500, 501–1000, 1001–5000, 5001–10,000, and >10,000 participants, respectively (Table 1).
Table 1.
Design Characteristics and Summary of the 145 Included Clinical Trials
| Category | Information | Number of Trials | Percentage of Total |
|---|---|---|---|
| Recruitment status | Recruiting | 76 | 52.4% |
| Not recruiting | 56 | 38.6% | |
| Authorized | 13 | 9.0% | |
| Allocation | Randomized | 99 | 68.3% |
| Not randomized | 12 | 8.3% | |
| Not provided | 34 | 23.4% | |
| Intervention model | Parallel Assignment | 109 | 75.2% |
| Not Parallel | 6 | 4.1% | |
| Single Group Assignment | 12 | 8.3% | |
| Adaptive | 2 | 1.4% | |
| Factorial Assignment | 3 | 2.1% | |
| Sequential Assignment | 2 | 1.4% | |
| Not provided | 11 | 7.6% | |
| Blinding | Double blinded | 25 | 17.2% |
| Single blinded | 13 | 9.0% | |
| Not blinded | 64 | 44.1% | |
| Not provided | 43 | 29.7% | |
| Phase | Phase 0 | 13 | 9.0% |
| Phase I | 2 | 1.4% | |
| Phase I/II | 1 | 0.7% | |
| Phase II | 20 | 13.8% | |
| Phase II/III | 19 | 13.1% | |
| Phase III | 58 | 40.0% | |
| Phase IV | 16 | 11.0% | |
| Not provided | 16 | 11.0% | |
| Number of group | 1 | 15 | 10.3% |
| 2 | 81 | 55.9% | |
| 3 | 23 | 15.9% | |
| 4 | 13 | 9.0% | |
| 5 | 9 | 6.2% | |
| 6 | 2 | 1.4% | |
| 9 | 2 | 1.4% | |
| Sample size | ≤100 | 70 | 48.3% |
| 101–500 | 39 | 26.9% | |
| 501–1000 | 10 | 6.9% | |
| 1001–5000 | 15 | 10.3% | |
| 5001–10,000 | 6 | 4.1% | |
| >10,000 | 5 | 3.4% | |
| Sample sizes | Median (IQR) | 108 (50–510) | |
| Sample sizes per Group | Median (IQR) | 50 (26–210) |
Clinical improvement time or rate and clinical recovery time or rate accounted for 29.7% and 11.0% of primary endpoints, respectively; 18.6% focused on virus-negative conversion time or rate, and 14.5% focused on all-cause mortality. Adverse outcome was the primary endpoint in 9.0% of studies, and other endpoints accounted for 17.2% (Table 2).
Table 2.
Primary Endpoints Employed in 145 Included Trials
| Category | Number of Trials | Percentage of Total |
|---|---|---|
| Clinical Improvement time or rate | 43 | 29.7% |
| Virus negative conversion rate or time | 27 | 18.6% |
| All-cause mortality | 21 | 14.5% |
| Clinical recovery time or rate | 16 | 11.0% |
| Adverse outcome | 13 | 9.0% |
| COVID-19 infection rate | 9 | 6.2% |
| Inflammatory marker | 5 | 3.4% |
| Hospitalization or ICU or death | 5 | 3.4% |
| Oxygen saturation | 4 | 2.8% |
| Inhospital time | 2 | 1.4% |
Antiviral Drugs Type Analysis
In all 145 antiviral drug clinical trials, 22 types of therapeutic interventions were used: 50 (34.5%) used lopinavir/ritonavir; 32 (22.1%) used favipiravir; 21 (14.5%) used remdesivir; 9 (6.2%) used arbidol; 4 (2.8%) used ASC09; 4 (2.8%) used azvudine; 3 (2.1%) used DAS181; 5 (3.4%) used danoprevir in combination with ritonavir or cobicistat; 6 (4.1%) used sofosbuvir in combination with daclatasvir, ledipasvir, or velpatasvir; 3 (2.1%) used emtricitabine in combination with tenofovir alafenamide or tenofovir disoproxil fumarate; and 7 (4.8%) used other drugs (Table 3).
Table 3.
Antiviral Drug Types in the 145 Included Clinical Trials
| Category | Number of Trials | Percentage of Total |
|---|---|---|
| Arbidol | 9 | 6.2% |
| ASC09 | 4 | 2.8% |
| Azvudine | 4 | 2.8% |
| Clevudine | 1 | 0.7% |
| CSA0001 | 1 | 0.7% |
| Danoprevir/Ritonavir | 2 | 1.4% |
| Danorevir sodium | 1 | 0.7% |
| Darunavir/cobicistat | 2 | 1.4% |
| DAS181 | 3 | 2.1% |
| Emtricitabine/Tenofovir Alafenamide | 2 | 1.4% |
| Emtricitabine/Tenofovir disoproxil fumarate | 1 | 0.7% |
| Favipiravir | 32 | 22.1% |
| Galidesivir | 1 | 0.7% |
| Interferon-Beta | 1 | 0.7% |
| Lopinavir/ritonavir | 50 | 34.5% |
| Oseltamivir | 1 | 0.7% |
| Remdesivir | 21 | 14.5% |
| Ribavirin | 2 | 1.4% |
| Sofosbuvir/daclatasvir | 4 | 2.8% |
| Sofosbuvir/ledipasvir | 1 | 0.7% |
| Sofosbuvir/Velpatasvir | 1 | 0.7% |
| Tenofovir | 1 | 0.7% |
Pharmacological Mechanism of Antiviral Drugs
Anti-Influenza Drugs
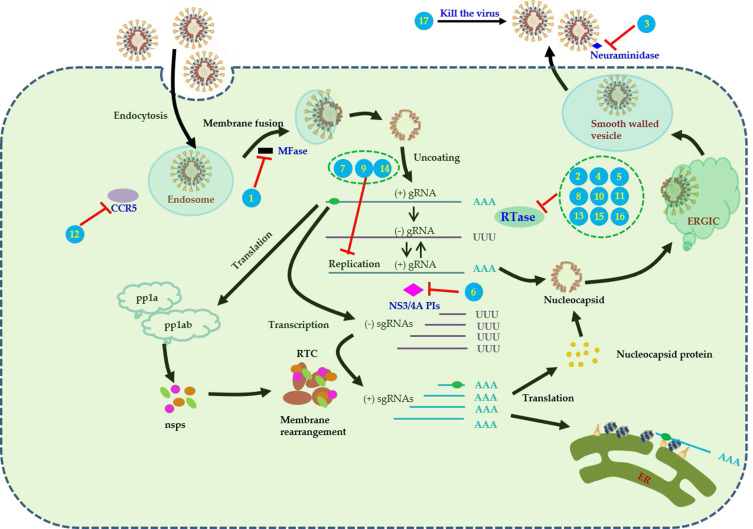
Arbidol, an anti-influenza drug, increases the acid stability of hemagglutinin (HA) and thus inhibits HA-mediated membrane fusion.9 Favipiravir (T-705) is known to inhibit RNA viruses’ RNA-dependent RNA polymerase (RdRp) selectively and potently.10 Oseltamivir acts by inhibiting neuraminidase in influenza A and B viruses11 (Table 4, Figure 3).
Table 4.
Results of Antiviral Drug Clinical Trials for COVID-19
| Number | Drug Name | Antiviral Mechanism | Indication | Reference |
|---|---|---|---|---|
| 1 | Arbidol | Non-nucleoside antiviral membrane fusion inhibitor | Influenza | [9] |
| 2 | Favipiravir | RNA-dependent RNA polymerase (RdRp) inhibitor | Influenza | [10] |
| 3 | Oseltamivir | Neuraminidase inhibitors | Influenza | [11] |
| 4 | Clevudine | Nucleoside and nucleotide reverse-transcriptase | HBV | [12] |
| 5 | Sofosbuvir/Daclatasvir | Nucleotide analog NS5B polymerase inhibitor/a putative NS5A inhibitor | HCV | [13,14] |
| 6 | Danoprevir/Ritonavir | NS3/4A PIs | HCV | [15] |
| 7 | Interferon-Beta | A broad‐spectrum antiviral drug | HBV infection | |
| 8 | Ledipasvir/sofosbuvir | HCV NS5A inhibitor/nucleotide analog NS5B polymerase inhibitor | HCV | [16] |
| 9 | Ribavirin | Interferes with the synthesis of viral mRNA | HCV | [17] |
| 10 | ASC09/ritonavir | Nucleoside and nucleotide reverse-transcriptase | HIV | Phase 2a(NCT04261907) |
| 11 | Azvudine | HIV reverse-transcriptase inhibitors | HIV | [18] |
| 12 | Darunavir/Cobicistat | The integrase inhibitors and chemokine receptor CCR5 antagonists. | HIV | [19] |
| 13 | Emtricitabine/tenofovir disoproxil | Reverse-transcriptase inhibitor | HIV | [20] |
| 14 | Lopinavir/ritonavir | HIV-1 protease inhibitor | HIV | [21] |
| 15 | Remdesivir | RNA-dependent RNA polymerase (RdRp) inhibitor | Ebola virus | [23] |
| 16 | Galidesivir | Inhibit viral RNA polymerase activity indirectly through non-obligate RNA chain termination | Broad-spectrum antiviral | [22] |
| 17 | CSA0001(LL-37 antiviral peptide) | Direct antimicrobial killing | Several viruses | [24] |
Figure 3.
Pharmacological mechanisms and therapeutic targets of antiviral drugs for COVID-19. Numbers in the figure represent different antiviral drugs. (1) arbidol, (2) favipiravir, (3) oseltamivir, (4) clevudine, (5) sofosbuvir/Daclatasvir, (6) danoprevir/ritonavir,(7) interferon-beta, (8) ledipasvir/sofosbuvir, (9) ribavirin, (10) ASC09/ritonavir, (11) azvudine, (12) darunavir/Cobicistat, (13) emtricitabine/tenofovir disoproxil, (14) lopinavir/ritonavir,(15) remdesivir, (16) galidesivir, (17) CSA0001 (LL-37 antiviral peptide).
Anti-Hepatitis B Virus and Anti-Hepatitis C Virus Drugs
Concerning drugs used against hepatitis B virus (HBV), clevudine demonstrates strong nucleoside analog reverse transcriptase inhibition.12 Concerning drugs used against hepatitis C virus (HBV), sofosbuvirm (an HCV NS5B nucleotide polymerase inhibitor13) and daclatasvir (a putative NS5A inhibitor14) are used in combination to treat chronic HCV infection. Moreover, some trials used danoprevir (ITMN-191/R7227; an HCV NS3/4A protease inhibitor [PI]15) and ledipasvir/sofosbuvir (an HCV NS5A and NS5B polymerase inhibitor16). In addition, a trial used ribavirin, which interferes with viral mRNA synthesis17 (Table 4, Figure 3).
Anti-HIV Drugs
ASC09 (TMC-310911), an HIV PI, has been established as effective for HIV infection treatment and is in the phase II of clinical trials (NCT00838162). Moreover, ASC09 in combination with ritonavir can enhance the antiviral activity of ASC09. Other anti-HIV drugs used include azvudine (functioning as a novel nucleoside reverse-transcriptase inhibitor that also exhibits anti-HIV activity18), darunavir/cobicistat (functioning as integrase inhibitors and chemokine receptor CCR5 antagonists19), emtricitabine (functioning as a reverse-transcriptase inhibitor typically combined with tenofovir disoproxil20), and lopinavir/ritonavir (comprising a fixed-dose PI combination employed in treating HIV-1 infection). Of the lopinavir/ritonavir combination, lopinavir represents the active component; nevertheless, when this component is applied alone, CYP3A4 is known to metabolize it extensively to produce low systemic concentrations. Accordingly, the potent CYP3A4 inhibitor ritonavir can be employed to enhance lopinavir’s systemic exposure21 (Table 4, Figure 3).
Other Antiviral Drugs
Galidesivir (BCX4430) can inhibit a broad spectrum of viruses, some of which are outlined as follows: flaviviruses, coronaviruses, bunyaviruses, arenaviruses, and paramyxoviruses.22 Moreover, remdesivir (GS-5734) exhibits broad-spectrum activity against RNA viruses;23 this drug is reported in the literature to be a 1′-cyano-substituted adenosine nucleotide analog prodrug. CSA0001, an LL-37 antiviral peptide, has been demonstrated to exhibit direct activity (executed through interaction with viral particles) and indirect activity (executed through the establishment of an antiviral state in the host cell) against several viruses24 (Table 4, Figure 3).
Efficacy for COVID-19 Antiviral Drugs Clinical Trials
In the open-label, nonrandomized controlled study lopinavir/ritonavir ChiCTR2000029600, a total of 35 and 45 patients were treated with favipiravir and lopinavir/ritonavir, respectively; they showed a virus clearance time of 4 and 11 days, respectively, indicating that favipiravir could shorten the virus clearance time.25 In ChiCTR2000030254, an open-label randomized controlled trial (RCT), recovery occurred in 71 of 116 patients treated with favipiravir (clinical recovery rate, 61.2%) and 62 of 120 patients treated with arbidol (clinical recovery rate, 51.7%). The between-group difference in clinical recovery rate on treatment day 7 was nonsignificant.26 A retrospective study comparing treatments with lopinavir/ritonavir and arbidol demonstrated the arbidol group to have a shorter duration of positive RNA test than did the lopinavir/ritonavir group.27 In the trial ChiCTR2000029308, another open-label RCT, 199 adults who were hospitalized due to severe COVID-19 were enrolled; of them, 99 underwent treatment involving lopinavir/ritonavir (constituting the drug group) and 100 underwent treatment involving standard care (constituting another group), but these groups did not demonstrate differences in time to clinical improvement.28 In NCT04276688, an open-label randomized trial pertaining to interferon β-1b, lopinavir/ritonavir, and ribavirin combination, the median time from the start of treatment until negative nasopharyngeal swab was noted to be significantly shorter in the group of patients subjected to the aforementioned combination than it was in the control group.29 A total of 237 adult patients diagnosed as having severe COVID-19 were included in NCT04257656, a randomized, double-blind, placebo-controlled study on remdesivir; of them, 158 and 79 were administered remdesivir and placebo, respectively, but demonstrated nonsignificant differences in clinical benefit30 (Table 5).
Table 5.
Summary of Antiviral Drug Clinical Trials for COVID-19
| Number | TrialID | Intervention | Study Design | Target Size | Primary Outcome Result | Reference |
|---|---|---|---|---|---|---|
| 1 | ChiCTR2000029600 | Favipiravir | Control Open-Label |
80 | A shorter viral clearance time was found for the FPV arm versus the control arm. | [25] |
| 2 | ChiCTR2000030254 | Favipiravir; Arbidol |
Randomized, controlled, open-label |
240 | Clinical recovery rate of Day 7 does not significantly differ between Favipiravir group and Arbidol group. | [26] |
| 3 | NA | Arbidol; Lopinavir/ritonavir |
Control | 50 | Patients in the arbidol group had a shorter duration of positive RNA test compared to those in the lopinavir/ritonavir group. | [27] |
| 4 | ChiCTR2000029308 | Lopinavir-Ritonavir | Randomized, controlled open-label |
199 | Treatment with lopinavir-ritonavir was not associated with a difference from standard care in the time to clinical improvement | [28] |
| 5 | NCT04276688 | Beta-1b, lopinavir–ritonavir, and ribavirin |
Randomized Open-label, |
127 | The combination group had a significantly shorter median time from start of study treatment to negative nasopharyngeal swab than the control group. | [29] |
| 6 | NCT04257656 | Remdesivir | Randomized, Double-blind, Placebo-controlled | 237 | Remdesivir use was not associated with a difference in time to clinical improvement. | [30] |
Summary of Antiviral Drugs for Different Stages of COVID-19
On the basis of treatment guidelines, we analyzed the different antiviral drugs used by COVID-19 stage. Treatment guidelines in China recommend early use of arbidol or ribavirin for patients in the mild-to-moderate stage. US guidelines recommend darunavir/cobicistat for severe cases and interferon-beta for critical cases. Treatment guidelines for lopinavir/ritonavir vary considerably, particularly among countries, but it can be used in the mild-to-moderate, severe, and critical stages. Egyptian guidelines recommend the use of oseltamivir for the mild-to-moderate, severe, and critical stages. Remdesivir is an antiviral drug that received worldwide attention and can be used to treat patients with COVID-19 in the mild-to-moderate, severe, and critical stages (Table 6).
Table 6.
| Antiviral Drugs | Stage of COVID-19 |
|---|---|
| Arbidol | Mild-to-moderate |
| Darunavir/cobicistat | Severe |
| Interferon-Beta | Critical |
| Lopinavir/ritonavir | Mild-to-moderate, Severe and Critical |
| Oseltamivir | Mild-to-moderate, Severe and Critical |
| Ribavirin | Mild-to-moderate |
| Remdesivir | Mild-to-moderate, Severe and Critical |
Discussion
The COVID-19 pandemic has endangered public health and safety seriously. However, research executed thus far has yet to identify effective antiviral drugs against this disease. Currently, COVID-19 treatment involves symptomatic comprehensive intervention as well as supportive therapy. During a pandemic, frontline medical personnel must step up their treatment approaches continually. Although some antiviral drugs have been verified to exhibit activity against SARS-CoV-2 in vitro, determining their clinical efficacy through clinical trials is essential.
Clinical trial registration ensures that researchers follow clinical trial transparency and crucial content availability with regard to the ethics of medical research and understand their obligations. With the progress of COVID-19 prevention and control research, registries have witnessed a continually increasing number of registered clinical trials: before March 2020, most of the relevant clinical trials were registered only in China, but as the outbreak became a pandemic, the number of such trials registered in other countries including Iran, the United States, and Spain is also increased.
In this study, of all types of trials, the proportion of RCTs was the largest. The present emergent COVID-19 pandemic, however, renders conducting double-blinded trials a difficult task; accordingly, we found that the proportion of such trials was small. Although a double-blind randomized trial produces least bias and although clinical studies afford the highest level of evidence,37 most of the currently retrieved trials were exploratory, with relatively small sample sizes.38 Nevertheless, for 26 and 5 studies on antiviral drugs, the sample sizes were >1000 (17.8%) and >10,000 (3.4%), respectively. Regarding the number of groups, 10.3% of the reviewed trials were determined to have included one group and the remaining 89.7% were determined to have included two or more groups. In addition to conventional parallel and group designs, new design types were used in the current clinical trials, including adaptive design (in JPRN-JapicCTI-205238 and ISRCTN50189673), factorial design (in PACTR202004893013257, ACTRN12620000445976, and NCT04403100), and sequential assignment (in NCT04372628 and ChiCTR2000033491). These clinical trials were conducted with the main features of the antiviral drugs.
The activity of drugs against SARS-CoV-2 has been demonstrated in vitro; thus, such drugs may be effective against COVID-19 in vivo, which can be confirmed through clinical trials. Accordingly, 17 antiviral drugs have been investigated in clinical trials thus far; these include RdRp inhibitors, HIV reverse-transcriptase inhibitors, HIV PIs, HCV NS3 PIs, and anti-influenza drugs.
Two favipiravir trials were performed in China: in the trial with lopinavir/ritonavir as the control, favipiravir demonstrated a shorter virus clearance time,25 and in that with arbidol as the control, no significant difference was noted in clinical recovery rate between favipiravir and arbidol.26 In a retrospective study, the group of arbidol-treated patients was determined to have a shorter duration of positive RNA test than did the group of lopinavir/ritonavir-treated patients.27 In a comparative study, compared with local standard care, lopinavir/ritonavir did not show differences in the time to clinical improvement.28 The median time from the start of treatment to negative nasopharyngeal swab was noted to be significantly shorter in the group receiving an interferon β-1b, lopinavir/ritonavir, and ribavirin combination than it was in the control group.29 Because all the aforementioned clinical trials were not double-blinded and used a small sample size, their results require verification through large-scale double-blind clinical trials. In the clinical trial of remdesivir for severe COVID-19, 453 patients were to be enrolled ideally; however, only 237 patients could actually be enrolled, and the results demonstrated no significant clinical benefit in adult patients with severe COVID-19.30
At present, the results of only a few registered clinical trials have been reported. Clinical trials including a large sample, a placebo, and randomization are still needed to verify antiviral drug efficacy. The WHO and its partners are launching an international randomized clinical trial, named “Solidarity,” to facilitate research on effective treatments for COVID-19 by reducing the design and implementation time by 80%.
A limitation of the present study is that clinical trials of antimalarial drugs and traditional Chinese medicine (TCM) were not included. Our previous research revealed that antimalarial drugs and TCM also account for a large proportion of clinical trials.8 In particular, natural products such as baicalein (ChiCTR2000033286) may play a key role in antiviral action and tissue repair; further clinical trials should test their efficacy. In the future, model-based meta-analysis (MBMA) may be employed to evaluate the efficacy of different antiviral agents employed in globally registered clinical trials. This is because MBMA can combine data from different types of clinical trials and concurrently probe the influence of various factors on efficacy parameters.39 Moreover, in the future, we aim to assess the effectiveness as well as safety of COVID-19 clinical trials so as to provide strong evidence for aiding the mitigation of COVID-19 in the affected countries.
Conclusion
By using ICTRP-registered data, we analyzed the study design characteristics of clinical trials executed on antiviral drugs for COVID-19 as well as the mechanisms of action and efficacies of the included drugs. Our results provide valuable information that can be used for guidance in future clinical trials on antiviral drugs for COVID-19 treatment and prevention.
Acknowledgments
We would like to thank all investigators who registered for clinical trials and the subjects who participated in these clinical trials. This study was supported by the Shanghai scientific and technological innovation action plan in 2017 (17401970900).
Abbreviations
ANZCTR, Australian New Zealand Clinical Trials Registry; ReBec, Brazilian Clinical Trials Registry; ChiCTR, Chinese Clinical Trial Register; CRiS, Clinical Research Information Service; ClinicalTrials.gov, ClinicalTrials.gov; CTRI, Clinical Trials Registry - India; RPCEC, Cuban Public Registry of Clinical Trials; EUCTR, EU Clinical Trials Register; DRKS, German Clinical Trials Register; IRCT, Iranian Registry of Clinical Trials; ISRCTN, International Standard Randomized Controlled Trial Number; JPRN, Japan Primary Registries Network; PACTR, Pan African Clinical Trial Registry; REPEC, Peruvian Clinical Trials Registry; SLCTR, Sri Lanka Clinical Trials Registry; TCTR, Thai Clinical Trials Register; NTR, The Netherlands National Trial Register.
Data Sharing Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://apps.who.int/trialsearch.
Disclosure
All authors declare no conflicts of interest in this work.
References
- 1.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Situation report - 117, coronavirus disease 2019 (COVID-19); 2020. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/situation-reports/.
- 3.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, He F, Zhou N, et al. Organ function support in patients with coronavirus disease 2019: Tongji experience. Front Med. 2020;14(2):232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu R, Wang L, Kuo HD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans T, Gülmezoglu M, Pang T. Registering clinical trials: an essential role for WHO. Lancet. 2004;363(9419):1413–1414. [DOI] [PubMed] [Google Scholar]
- 8.Huang JH, He YC, Su QM, Yang J. Characteristics of COVID-19 clinical trials in China based on the registration data on ChiCTR and clinicaltrials.gov. Drug Des Devel Ther. 2020;14:2159–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brancato V, Peduto A, Wharton S, et al. Design of inhibitors of influenza virus membrane fusion: synthesis, structure-activity relationship and in vitro antiviral activity of a novel indole series. Antiviral Res. 2013;99(2):125–135. [DOI] [PubMed] [Google Scholar]
- 10.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubareva L, Mohan T. Antivirals targeting the neuraminidase. Cold Spring Harb Perspect Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jang YO, Quan X, Das R, et al. High-dose clevudine impairs mitochondrial function and glucose-stimulated insulin secretion in INS-1E cells. BMC Gastroenterol. 2012;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herbst DA Jr, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013;22(4):527–536. [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Ma H, Hang JQ. The hepatitis C virus NS5A inhibitor (BMS-790052) alters the subcellular localization of the NS5A non-structural viral protein. Virology. 2011;414(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Y, Andrews SW, Condroski KR. Discovery of danoprevir (ITMN-191/R7227), a highly selective and potent inhibitor of hepatitis C virus (HCV) NS3/4A protease. J Med Chem. 2014;57(5):1753–1769. [DOI] [PubMed] [Google Scholar]
- 16.Scott LJ. Ledipasvir/sofosbuvir: a review in chronic hepatitis C. Drugs. 2018;78(2):245–256. [DOI] [PubMed] [Google Scholar]
- 17.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang RR, Yang QH, Luo RH, et al. Azvudine, a novel nucleoside reverse transcriptase inhibitor showed good drug combination features and better inhibition on drug-resistant strains than lamivudine in vitro. PLoS One. 2014;9(8):e105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lascar RM, Benn P. Role of darunavir in the management of HIV infection. HIV AIDS (Auckl). 2009;1:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corado KC, Daar ES. Emtricitabine + tenofovir alafenamide for the treatment of HIV. Expert Opin Pharmacother. 2017;18(4):427–432. [DOI] [PubMed] [Google Scholar]
- 21.Corbett AH, Lim ML, Kashuba AD. Kaletra (lopinavir/ritonavir). Ann Pharmacother. 2002;36(7–8):1193–1203. [DOI] [PubMed] [Google Scholar]
- 22.Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508(7496):402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchesnokov EP, Feng JY, Porter DP, Götte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed A, Siman-Tov G, Keck F, et al. Human cathelicidin peptide LL-37 as a therapeutic antiviral targeting Venezuelan equine encephalitis virus infections. Antiviral Res. 2019;164:61–69. [DOI] [PubMed] [Google Scholar]
- 25.Cai Q, Yang M, Liu D, et al. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Zhang Y, Huang J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical. Trial MedRXIV. 2020. [Google Scholar]
- 27.Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung IF, Lung KC, Tso EY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, Phase 2 trial. Lancet. 2020;395(10238):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Zhang M, Yin L, et al. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int J Antimicrob Agents. 2020;56(2):106080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobaiqy M, Qashqary M, Al-Dahery S, et al. Therapeutic management of patients with COVID-19: a systematic review. Infect Prev Pract. 2020;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falavigna M, Colpani V, Stein C, et al. Guidelines for the pharmacological treatment of COVID-19. The task-force/consensus guideline of the Brazilian Association of Intensive Care Medicine, the Brazilian Society of Infectious Diseases and the Brazilian Society of Pulmonology and Tisiology. Rev Bras Ter Intensiva. 2020;32(2):166–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan L, Jiang S, Yang X, Wang Z, Yang C. COVID-19 drug treatment in China. Curr Pharmacol Rep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta Y, Chaudhry D, Abraham OC, et al. Critical care for COVID-19 affected patients: position statement of the indian society of critical care medicine. Indian J Crit Care Med. 2020;24(4):222–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rochwerg B, Agarwal A, Zeng L, et al. Remdesivir for severe COVID-19: a clinical practice guideline. BMJ. 2020;370:m2924. [DOI] [PubMed] [Google Scholar]
- 37.Mad P, Felder-Puig R, Gartlehner G. Randomised controlled trials. Wien Med Wochenschr. 2008;158(7–8):234–239. [DOI] [PubMed] [Google Scholar]
- 38.Huang JH, Su QM, Yang J, et al. Sample sizes in dosage investigational clinical trials: a systematic evaluation. Drug Des Devel Ther. 2015;9:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boucher M, Bennetts M. The many flavors of model-based meta-analysis: part I – introduction and landmark data. CPT Pharmacometrics Syst Pharmacol. 2016;5(2):54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]