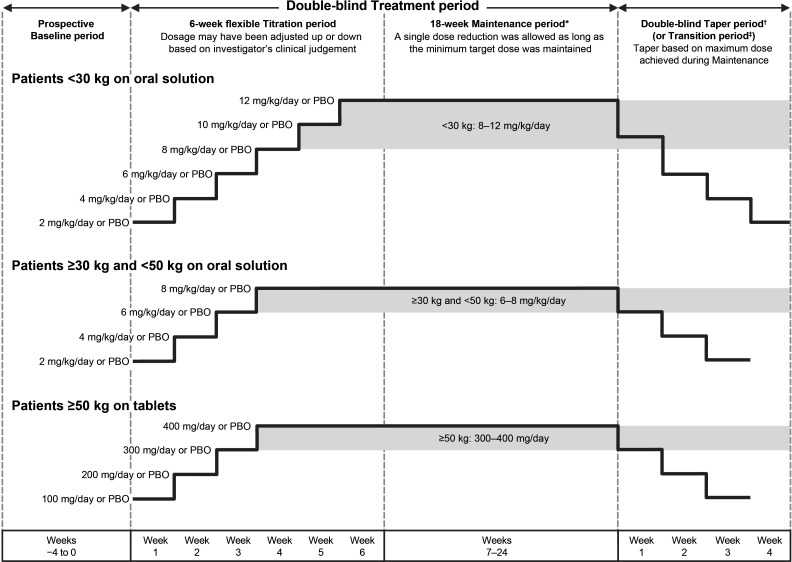
Figure 1.
Trial design. *Patients were required to achieve and maintain a minimum lacosamide (or matching PBO) dose for at least the final 3 days of week 6 to be eligible for entry into the maintenance period. †The highest possible dose per body weight category is shown for each taper period week. ‡Patients on lacosamide remained on their maintenance dose at entry into the transition period (as indicated by the grey background box), whereas patients in the PBO group initiated lacosamide in a double-blind fashion. On completion of the transition period, eligible patients entered the open-label extension on a weight-based dose (<30 kg: 10 mg/kg/day; ≥30–<50 kg: 8 mg/kg/day; ≥50 kg: 400 mg/day). PBO, placebo.