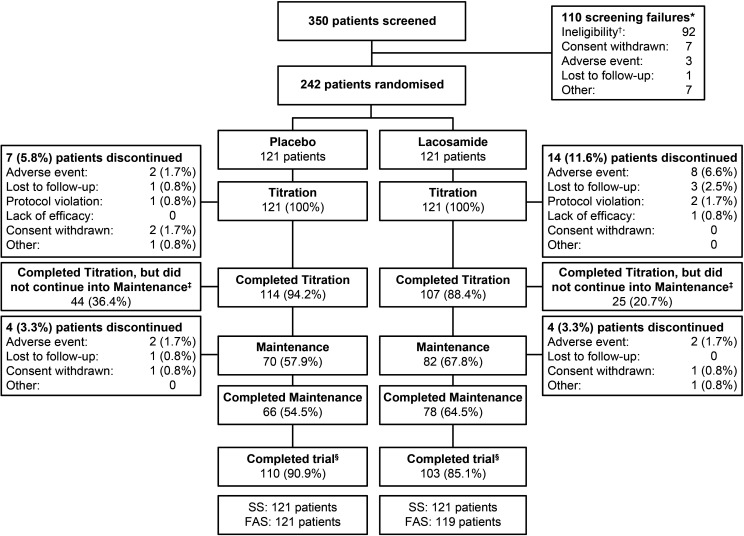
Figure 2.
Patient disposition. *Two of these patients were successfully rescreened and randomised into the trial; †37 patients were baseline failures because of their PGTCS frequency during the combined baseline; ‡41/44 patients on placebo and 22/25 patients on lacosamide did not continue into maintenance because they had a second PGTCS during titration, one patient on lacosamide was labelled as a completer due to a site error, and the five remaining patients did not continue into maintenance because the 125th event had occurred in the trial; §Patients who met a protocol-defined endpoint (completion of ≥6 weeks of the treatment period and occurrence of two or more PGTCS, completion of 24 weeks of the treatment period without occurrence of two PGTCS, or the 125th event occurred in the trial). FAS, full analysis set; PGTCS, primary generalised tonic-clonic seizure; SS, safety set.