Figure 8.
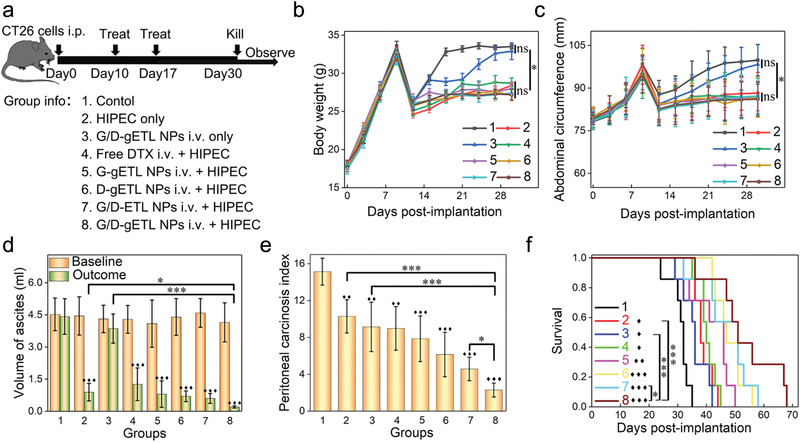
Characterization of the therapeutic benefits of NP treatment in CT26‐derived mPC xenografts. a) Schematic illustration of the experimental design. b) Change of body weight with time. c) Change of abdominal circumference with time. d) Ascites baseline and outcome of animals. e) Outcome of tumor burden for animals in term of peritoneal carcinosis index (PCI). f) Kaplan‐Meier survival analysis of mice received the indicated treatment. Median survival time was used for statistical analysis. The data was presented as the mean ± SD, One‐way ANOVA was used to determine statistical differences (♦, p < 0.05, ♦♦, p < 0.01, ♦♦♦, p < 0.001, compared with control group; *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant, compared with the indicated groups).
