Figure 2.
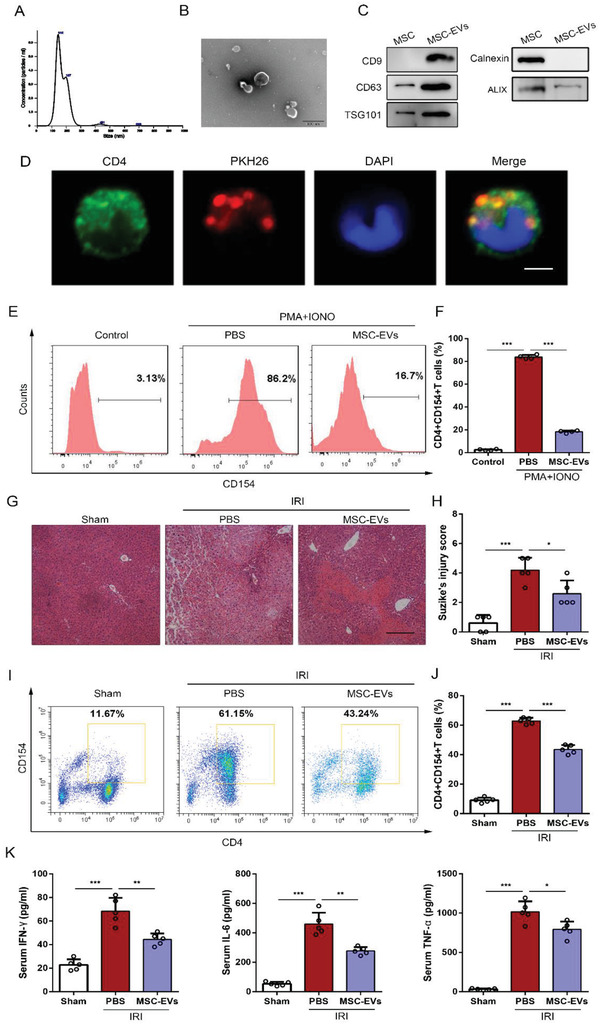
UC‐MSC‐EVs improve liver IRI and regulate CD154 expression on CD4+ T cells in vitro and in vivo. Characterization of harvested UC‐MSC‐EVs. A) Nanoparticle tracking analysis was performed to detect the diameter quantitation of UC‐MSC‐EVs. B) The spheroid morphology and size of UC‐MSC‐EVs were investigated under SEM. Original scale bar: 0.2 µm. C) Western blot analysis was conducted to detect the specific vesicle‐related markers CD63, CD9, ALIX, TSG101, and calnexin in UC‐MSC‐EVs. D) The CD4+ T cells were stimulated by PMA and ionomycin and treated with PKH26‐labeled UC‐MSC‐EVs for 6 h, a representative fluorescence image of UC‐MSC‐EVs (red) uptake in CD4+ T cells (green) was detected and photographed using confocal microscopy (bar = 2 µm). In vitro experiments, primary CD4+ T cells isolated from the spleen of mice were stimulated by PMA and ionomycin, and co‐cultured with PBS or UC‐MSC‐EVs. E) Flow cytometry analyses of CD154 expression on CD4+ T cells of each treatment group. F) Quantification of membranous CD154 expression of CD4+ T cells. The data are presented as the means ± SEM (n = 3 per group). Mice that underwent liver IRI and were treated with UC‐MSC‐EVs or PBS were sacrificed at 6 h after reperfusion. G) Representative sections of livers stained with H&E from three groups after different treatments (bar = 200 µm). H) The severity of liver injury was evaluated from histological section and scored according to Suzike's injury criteria. The data are expressed as the means ± SEMs (n = 5 per group). I,J) Flow cytometry analyses of CD4+CD154+ T cells in intrahepatic CD3‐positive mononuclear cells of each treatment group. Statistical analyses of the percent of CD4+CD154+ T cells in each group. Data are presented as the means ± SEM (n = 5 mice per group). K) The levels of IFN‐γ, IL‐6, and TNF‐α in serum from each group were measured using ELISA assay. Data are presented as the means ± SEM (n = 5 mice per group). *p < 0.05, **p < 0.01, ***p < 0.001 (all p values were obtained by one‐way ANOVA).
