Abstract
Advanced Therapy Medicinal Products (ATMPs) comprise novel cell, tissue and gene therapies and offer the potential of durable remissions for diseases where there is a high unmet clinical need. Once considered a niche area of academic research, ATMPs now represent one of the fastest-growing areas of clinical development. The field has seen a rapid expansion of academic and commercial entities successfully translating ATMP research into the clinic. This is reflected in projection that the global gene and cell therapy market will be worth US $11.96 billion by 2025. However, these treatments are complex to deliver and frequently do not fit naturally into established healthcare systems. In the United Kingdom (UK) there has been a long-standing interest in ATMP research and, in order to meet the ambition to act as an international hub of activity for delivery of ATMPs, a collaborative network of Advanced Therapy Treatment Centres (ATTCs) has been established. This review explores the challenges of delivery in the clinical setting, focussing on one form of ATMP, Adoptive Cell Therapy (ACT). We describe the strategy being implemented in the UK to optimise the roll-out of these exciting new therapies.
Keywords: Advanced Therapy Medicinal Product, (ATMP), Advanced Therapy Treatment Centre (ATTC), ACT, T cell, CAR-T, CD19, TIL, TCR T cell
Introduction
Rapid commercial development of chimeric antigen receptor T-cell (CAR-T) therapy in the setting of acute B-cell lymphoblastic leukaemia (B-ALL) and diffuse large B-cell lymphoma (DLBCL)1–5 has catapulted Advanced Therapeutic Medicinal Products (ATMPs) onto the global stage. There are currently over 4500 gene therapy trials registered on clinicaltrials.gov,6 with projections that the international cell and gene therapy market will be worth up to US $11.96 billion by 2025.7 However, the branch of ATMP therapy to which CAR-T belongs, adoptive cell therapy (ACT) with immune effector cells (IECs), has roots in the solid tumour setting with treatment protocols established during the 1980s at the National Cancer Institute.8 These were focussed primarily on tumour infiltrating lymphocyte (TIL) therapy in melanoma, and formed the basis of the methodology used at scale today. Two CD19-directed CAR-T cell therapies were approved by both the United States (US) Food and Drug Administration (FDA) and European Medicines Agency (EMA) in 2018 and are currently being administered at multiple UK clinical sites.9 Regulatory approval of these products has heightened commercial interest in the field to improve efficacy, reduce toxicity and expand this technology to a wider oncology patient population.10 This interest is reflected in a rapidly increasing number of clinical trials. As of December 2019, there are 852 open clinical trials of T-cell-based ACTs around the world.6 In order to deliver this increasing activity of both commercial products and clinical trials in the UK, it is accepted that for the infrastructure required to deliver ATMPs, and ACT in particular, protocols will need to evolve significantly.11 If the UK is to realise the opportunity to be internationally leading in the field, administration can no longer be conducted solely by small independent groups; it requires collaboration, as well as integration into the mainstream UK healthcare system. The complex nature of the technology, highlighted not least by the number of acronyms surrounding it (Table 1), demonstrates the requirement for consensus as well as innovation in order to maximize the potential of ACT globally.
Table 1.
Definitions of key entities within the field.
| Acronym | Full term | Description |
|---|---|---|
| ATMP | Advanced therapeutic medicinal product | A medicinal product for human use that is any of: gene therapy, somatic cell therapy, tissue engineered product |
| IEC | Immune effector cell | A cell that has differentiated into a form capable of modulating or effecting a specific immune response |
| ACT | Adoptive cell therapy | Involves administration of a cell product to patients with therapeutic intention. Including but not limited to CAR-T, TCR and TIL therapy. |
| CAR-T | Chimeric antigen receptor T cell | T lymphocytes (T cells) that have been genetically modified to express an artificial chimeric receptor that can recognise a specific antigen and cause activation of the T cell through a signalling domain most commonly CD3ζ |
| TCR T cell | T-cell receptor T cell | T lymphocytes (T cells) that have been genetically modified to express an transgenic T cell that can recognise a specific antigen |
| TIL | Tumour infiltrating lymphocyte | T cells that infiltrate solid tumours and are naturally reactive to autologous tumour antigens |
Clearly this poses significant challenges. Whilst UK-based hospitals and clinical sites are well versed in the adoption of standard oncology drug, devices and services, the complexities involved in implementing an ACT service lie outside established workflows. These products are not simply delivered to hospital pharmacies in a vial for re-constitution, but involve complex scheduling and transportation logistics. To ensure successful adoption, the challenge for UK health systems is to anticipate bottlenecks within current practices and devise robust methods to overcome them. The reward of successful implementation will be significant; providing a unique opportunity for interested clinicians to influence the clinical infrastructure that will be developed; to ensure that they are viable and sustainable; and to ensure that UK clinical sites can capitalise on the expansion of ACT technologies by attracting commercial partners for product delivery. Whilst this paper focusses on ACT, it is clear that many of the challenges are mirrored in the challenges of all forms of ATMPs, including tissue engineering and viral gene therapy, for example, bottlenecks in vector manufacture infrastructure deficiencies at clinical sites and institutional readiness to safely deliver these complex drugs. This paper, taking ACT as one example, therefore reflects many of these wider challenges across the whole ATMP field.
ACT delivery requires investment, special clinical infrastructure and regulation
Developing a service to support ACT-based therapies requires significant investment both by national healthcare providers and local clinical sites and requires careful appraisal. Key initial considerations include firstly, who will develop and lead the service? For example, will it be adopted into already established Haematopoietic Stem Cell Transplant (HSCT) programs irrespective of disease type being treated; or run by individual disease site specific oncologists; or within a unified ‘ACT Delivery’ Team. Secondly, assessment of projected numbers of patients to inform the service development strategy and investment requirements going forward.
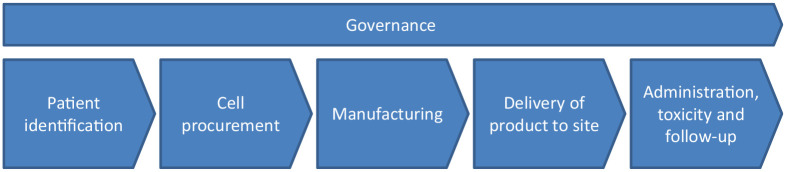
Delivering an ACT service involves an entirely new model of work, and identifying gaps in current treatment pathways will aid understanding of the clinical services and infrastructure that will be needed. The treatment pathway is common to most ACT products (Figure 1) and considers the needs of the patient, clinicians and product manufacturers. The remainder of this review will explore the challenges and potential solutions in each of these areas for delivery of a successful ACT program.
Figure 1.
Overview of UK treatment pathway for ACT demonstrating six broad stages within which key challenges need to be addressed for safe delivery.
ACT, adoptive cell therapy; UK, United Kingdom.
Clinical pathway for patients in the UK receiving ACT
Patient identification
The first step in any treatment pathway is the identification of patients and an evaluation of their suitability for therapy, either within or outside of a clinical trial setting. This includes an assessment of the patient’s physical fitness to receive ACT and also a series of investigations to confirm eligibility, some of which may be outside the scope of current practice at some clinical sites. CAR-T and TCR ACT products that contain modified receptors targeting a specific antigen may require confirmation of the antigen’s expression in target tissue through a new tumour biopsy sent to an external laboratory to confirm eligibility. Human leukocyte antigen (HLA) subtyping may also be required.
Patients who are potentially suitable for CD19 CAR-T are currently discussed at a national panel and manufacturing slots are allocated. As demand intensifies, slot allocation will be of increased importance, and managing both the patient’s expectation and fitness to receive treatment may prove challenging. This also translates to therapies received in clinical trials where cell manufacturing capabilities are likely to be reduced and therefore competition all the more severe.
Cell procurement
For the currently approved CD19 CAR-T therapies and other peripherally derived gene modified ACT products in clinical development, clinical sites act as cell procurement centres. Cells are harvested via apheresis prior to their shipping to Good Manufacturing Practice (GMP)-licenced third parties for manufacturing. As demand increases, outsourcing of cell procurement to commercial apheresis facilities may be required. This requires both complex contract arrangements between clinical and manufacturing sites to allocate responsibilities and appropriate regulatory approvals. The procurement of biological materials required for the production of ACT requires a license, which in the UK is regulated by both the Human Tissue Authority (HTA) and the Medicines and Healthcare products Regulatory Agency (MHRA) under a Blood Establishment Authorisation. Licensing is essential to ensure suitable consent is obtained, appropriate screening of donors for infectious disease is performed and full traceability of the biological material from the donor to the manufacturing facility is in place. In all licensed establishments, the HTA procurement licence is overseen by a Designated Individual (DI), often affiliated with the haematology department as part of the HSCT program. This DI may not have experience or knowledge of procurement from other types of ACTs such as the surgical specimens required to isolate TILs. Surgical centres may be remote from the clinical site with experience in delivering ACT therapy. As such, there will be a need for expansion in the responsibilities and capacity of the DI, who will potentially need to oversee operating theatres and surgical staff who have never worked under such a regulated quality system.
Manufacturing
Historically, ACT products delivered in small academic, single site, early phase clinical studies, were manufactured in facilities either within, or closely affiliated with, the clinical site. As these academic enterprises have commercialised, manufacturing capacity has increased to support larger multi-site studies, and therefore it is less likely that manufacturing of an ACT product will take place on the clinical site. This is particularly true of commercial CD19 CAR-T products. Despite clinical sites not being directly involved in manufacturing, they will still need an agreement on how to safeguard the chain of custody and chain of identity of the procured cells from extraction to infusion, including that manufacturing is taking place under the appropriate regulatory oversight.
Delivery of product to and from clinical site
The transportation and storage of starting materials and returned manufactured products will also pose logistical challenges and may demand specialist infrastructure. Whilst most standard medicines delivered to clinical sites become the responsibility of pharmacy units, and are stored within their department, ACTs may be delivered for immediate administration, or, more commonly, arrive cryopreserved. Such products will require cold-chain transportation and, again, appropriate quality-assurance measures. Short-term storage of cryopreserved products are likely to occur in HSCT laboratories, which already have processes in place for handling products in vapour phase nitrogen in accordance with regulatory standards. Capacity for such storage, especially for genetically modified products and in smaller HSCT units, could rapidly become a limiting factor, requiring investment in expensive equipment.
Administration and toxicity management
In the UK, CD19 CAR-T product administration has been adopted by existing HSCT units and managed by accredited haematologists. ACT delivery in the solid tumour setting is currently confined to clinical trials and therefore the logistical considerations are more complex. Should treatment also be managed in HSCT units where capacity is likely to rapidly become a limiting factor, standard oncology inpatient wards or designated early phase clinical trial inpatient units? Governance and accreditation issues (discussed below) can aid these decisions where there is the potential of specialist ACT units managing patients irrespective of tumour type.
Regardless of treatment location, the ability to recognise and promptly manage treatment-related complications associated with ACT is key to safe practice. Cytokine release syndrome and neurological complications are commonly reported with CD19 CAR T therapy.12 Caused by the release of inflammatory cytokines by both the infused CAR-T cell and bystander immune cells, CRS is a systemic illness that closely mimics sepsis. Patients typically develop a fever, which may be followed by haemodynamic instability, capillary leak and multiorgan failure in severe cases. Whilst mild cases can be managed with supportive measures such as intravenous fluids, patients with severe CRS may require cytokine-directed therapy with the anti-IL6 monoclonal antibody tocilizumab, corticosteroids and even intensive care measures. Rapid recognition and initiation of treatment of CRS by appropriately trained staff is paramount.
Management of patients receiving ACT is truly multidisciplinary, involving nursing staff, oncology and haem-oncology medical colleagues, acute and intensive care physicians and neurologists. There is a clinical need to have access to urgent specialist medical input, and out of hours imaging is essential. This may be particularly difficult for stand-alone cancer centres where these services may not be available onsite. Clinical sites will be required to demonstrate that attending medical staff are trained to identify and manage treatment-related complications and to ensure that pharmacy supplies of medicines such as the anti-IL6 monoclonal antibody, tocilizumab, are replenished.
In addition to acute inpatient management, ensuring appropriate post treatment clinical follow up of patients receiving ACT is also of importance. This will be critical for facilitating future health economic analysis exploring not just long-term survival but also quality of life assessments and safety follow up for late toxicities, which may be potentially significant. The European Society for Blood and Marrow Transplantation patient registry is well established in the field of HSCT, and is now collecting data on both commercial and investigational CAR-T products.13
Quality governance considerations
Given the above described administrative and logistical challenges of delivering ACT alongside the potential for significant toxicity, it is clear that regulatory guidance is essential. In Europe, HSCT programs often function under JACIE [Joint Accreditation Committee ISCT (International Society for Cellular Therapy) and EBMT (European Group for Blood & Marrow Transplantation)] accreditation. JACIE accreditation is not legislative; however, it is internationally recognised as a validated and effective system of quality management that impacts on survival outcomes and is considered best practice throughout the HSCT community.14 Accreditation is awarded following inspection against compliance with an internationally recognised set of standards, which now include specific guidance for immune effector cells (IEC).15 In the UK, following the approval of commercial CD19 CAR-T products in 2018, the National Health Service (NHS) adopted this model of ensuring effective governance by mandating that the first wave of NHS providers must hold JACIE accreditation.
In the US, a collaboration between the Foundation of Accreditation of Cellular Therapy (FACT), the American Society of Gene and Cellular Therapy (ASGCT), the Society of Immunotherapy of Cancer (SITC) and representatives from the academic, clinical and cell manufacturing fields, has developed quality standards and an accreditation program for IEC, which include T cell therapies.16 These FACT IEC standards display significant overlap with those required for HSCT programs but are separate, reflecting the reality that different clinical teams and infrastructure may be responsible for delivering ACT across clinical sites. Attaining FACT IEC accreditation following a comprehensive inspection, which is reviewed every 3 years, confirms that the clinical delivery site is committed to delivering ACT therapy according to internationally accepted quality standards. Obtaining accreditation whether FACT IEC or JACIE, however, is complex and requires clinical sites to demonstrate significant understanding, oversight and competence in all areas of the treatment pathway.
The UK’s response
In response to the recent advances in ATMP development and as part of its Industrial Strategy Policy, the UK Government has made significant investment to create infrastructure and capabilities to support the development and adoption of ATMPs and ACTs in particular. In 2018, £30 million from Innovate UK was made available to create a national network of advanced therapy treatment centres (ATTC) with the aim of pioneering partnerships between NHS clinical centres, academia and the commercial sector to scale up activity and clinical delivery across the whole patient pathway in ATMPs. This ATTC network was to be co-ordinated by the Cell and Gene Therapy Catapult.17
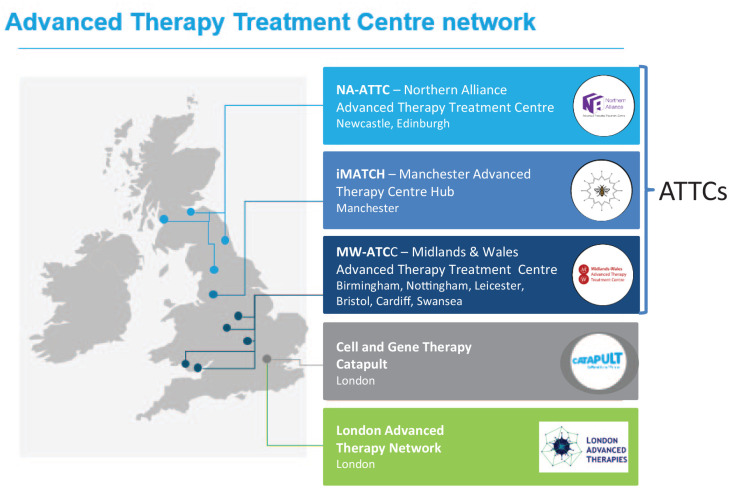
Three ATTCs spread geographically across the UK were awarded Innovate UK funding in 2018 (Figure 2): Innovate Manchester Advanced Therapy Centre Hub (iMATCH); Midlands and Wales Advanced Therapy Treatment Centre (MW-ATTC) and Northern Alliance Advanced Therapies Treatment Centre. In addition, the London Advanced Therapies (LAT) and the Advanced Therapies Network (ATN) have been funded by Research England’s Connecting Capabilities fund. Both aim to support networking opportunities and knowledge exchange between academic organisations, life sciences companies and investors in the London area.
Figure 2.
The ATTC network. The three successful ATTC networks are spread geographically across the UK. LAT has been funded by Research England’s Connecting Capabilities fund.
ATTC, advanced therapy treatment centre; LAT, London Advanced Therapies; UK, United Kingdom.
Although separate entities, the ATTCs are working collaboratively across five key themes:
1. Manufacturing and the supply chain
This includes production of a comprehensive process development template that captures all the steps in ATMP production, thus allowing evaluation of manufacturing requirements. The SAMPLE (standard approach to ATMP tissue collection) project led by iMATCH, is a pan-ATTC venture aiming to standardise the way cell and gene samples are procured. Developed in anticipation of the widely held view that as the number of ATMP therapies grows there will be an increased need to streamline the way we procure samples, whether from blood or tissue. Workstreams are exploring areas of standardisation and harmonisation across apheresis, tissue collection and cryopreservation.
2. ATMP patient pathway
The MW-ATTC is creating a patient and product process map to capture the complex steps involved from initial referral though to follow up to identify areas for development. This will feed into their model framework for a Therapy Acceleration Programme for Cellular Therapies, which, through dissemination across clinical sites, will increase the speed of delivery of clinical trials. Clinical trial design and implementation is a further focus for the NA-ATTC. They aim to foster best practice through the formation of a Clinical Advisory Group, ensuring a patient-centred approach to safe and effective trial delivery.
3. Education and training
Increasing ATMP awareness, education and training are key objectives of the ATTC network as a whole to establish best practice for safe and delivery of ATMPs to patients. The iMATCH team have developed a Pan-Manchester education steering committee responsible for delivering local, regional and national ATMP education programs. Competency of medical staff and adhering to JACIE standards has been achieved though focussed practical ATMP education sessions. A range of educational materials including e-learning modules are developed for national distribution and working with the University of Manchester, an ATMP MSc course has been launched, with the first students accepted this year.
4. Informatics
Across the ATTC network, novel digital solutions are being developed to enable electronic sample and ATMP traceability both within the manufacturing supply chain and within clinical sites. Innovative systems of clinical data capture designed specifically to address complexity of ATMP therapies are being developed to create secure, user-friendly scalable database for clinical trial data, follow up and outcomes. Informatics will also be utilised to enhance patient engagement through the development of an app to inform and involve patients in their ATMP journey.
5. Economic evaluation and reimbursement
Taking ATMPs from a successful clinical trial to UK market authorisation is complex, involving engagements with European and National commissioning and regulatory authorities. Work packages across the NA and MW-ATTCs will establish requirements to inform the development of models to aid commissioning decisions. Furthermore, they aim to provide guidance to ATMP developers on robust applications to regulatory and commissioning bodies, facilitating clinical adoption of ATMP products.
Conclusion
The approval of two commercial CD19 CAR-T cell products is a significant landmark for ACT development and heralds that the technology is finally delivering on its considerable promise. Adopting ACT and other ATMPs into existing NHS services is undoubtedly a challenge, with many obstacles to overcome. The UK government has prioritised ATMP development and delivery and has taken exciting, innovative and financed steps to achieve this goal through the establishment of the ATTC network. Aided by this, NHS clinical sites have been actively putting in place the required infrastructure and are now delivering ATMPs at scale within both the standard-of-care and research settings.
Acknowledgments
We would like to acknowledge and thank all members of the UK ATTC network for their contribution to this work.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MD is funded as part of The National Institute for Health Research funded Manchester Clinical Research Facility.
Conflict of interest statement: FT is the Director of iMATCH ATTC and Clinical lead for SAMPLE project. She has consultancy agreements with BMS, Achilles Therapeutics, Kite Gilead, GSK, Zelluna Immunotherapy and research funding from Novartis. MD has consultancy agreement with Kite Gilead. MP has no conflicts of interest to declare.
ORCID iD: Fiona C Thistlethwaite  https://orcid.org/0000-0002-4832-7008
https://orcid.org/0000-0002-4832-7008
Contributor Information
Manon Pillai, The Christie NHS Foundation Trust, Manchester, UK.
Michelle M. Davies, The Christie NHS Foundation Trust, Manchester, UK
Fiona C. Thistlethwaite, Department of Medical Oncology, The Christie NHS Foundation Trust, Wilmslow Road, Manchester, M20 4BX, UK; Division of Cancer Sciences, Faculty of Biology Medicine and Health, The University of Manchester, Manchester, UK.
References
- 1. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 4. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378: 439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CAR-T cell therapy gets breakthrough status. Nat Biotechnol 2014; 32: 851.25203014 [Google Scholar]
- 6. ClinicalTrials.gov. U.S. National Library of Medicine, https://clinicaltrials.gov/ct2/results?cond=T+cell+therapy (accessed 1 December 2019).
- 7. BIS Reseasrch. Global cell and gene therapy market: focus on products, applications, regions and competitive landscape – analysis and forecast, 2019–2025, https://bisresearch.com/industry-report/cell-gene-therapy-market.html
- 8. Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 33: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 9. NHS England CAR-T therapy approval and service specifications, https://www.england.nhs.uk/cancer/cdf/car-t-therapy/ (accessed 1 December 2019).
- 10. Azizi AA, Pillai M, Thistlethwaite FC. T-cell receptor and chimeric antigen receptor in solid cancers: current landscape, preclinical data and insight into future developments. Curr Opin Oncol 2019; 31: 430–438. [DOI] [PubMed] [Google Scholar]
- 11. Association of the British Pharmaceutical Industry. Advanced therapies manufacturing action plan, https://www.abpi.org.uk/publications/advanced-therapies-manufacturing-action-plan/ (accessed 1 December 2019).
- 12. Hirayama AV, Turtle CJ. Toxicities of CD19 CAR-T cell immunotherapy. Am J Haematol 2019; 94: S42–S49. [DOI] [PubMed] [Google Scholar]
- 13. European Society for Bone and Marrow Transplantation, https://www.ebmt.org/ebmt-patient-registry (accessed 1 December 2019).
- 14. Snowden J, McGrath E, Duarte R, et al. JACIE accreditation for blood and marrow transplantation: past, present and future directions of an international model for healthcare quality improvement. Bone Marrow Transplant 2017; 52: 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. FACT-JACIE international standards for hematopoietic cellular therapy product collection, processing, and administration. 7th ed, https://www.ebmt.org/sites/default/files/2018-06/FACT-JACIE%207th%20Edition%20Standards.pdf (accessed 28 October 2019).
- 16. FACT Immune Effector Cell Standard, http://www.factwebsite.org/iecstandards/ (accessed 1 October 2019).
- 17. The ATTC Network, https://www.theattcnetwork.co.uk (accessed 1 December 2019).