Figure 3.
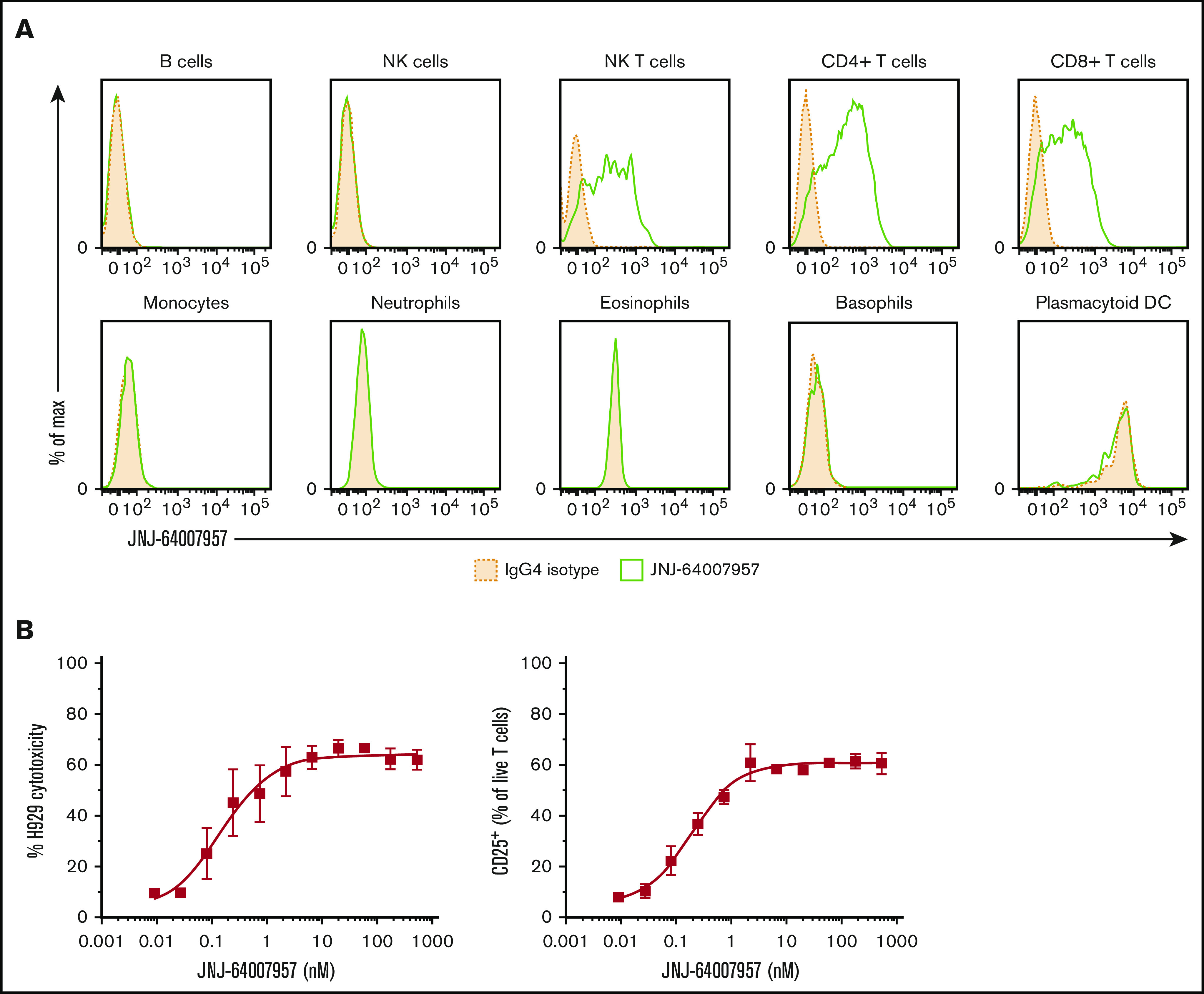
Teclistamab activity in a whole blood assay. (A) Whole blood from 3 healthy human donors was stained with teclistamab. Staining intensity for 1 representative donor is shown in the panels, where solid green lines represent teclistamab, and filled dotted lines are the corresponding isotype. No BCMA expression was observed on lymphocytes (B cells were gated by using CD19 marker), monocytes, granulocytes, NK cells, or plasmacytoid dendritic cells (DCs) in 3 healthy donors. In contrast, the antibody bound to T cells (CD4+ and CD8+) and NK T cells as expected because they express CD3. (B) Dose–response curve of H929 cytotoxicity and T-cell activation as measured by percent CD25+ T-cell population in whole blood after 48-hour incubation with teclistamab. The graphs depict the mean (± standard error of the mean [SEM]) of 6 individual donors.
