Figure 8: Inhibition of TrxR by acrolein and subsequent reversal of acrolein inhibition using H2O2 and imidazole.
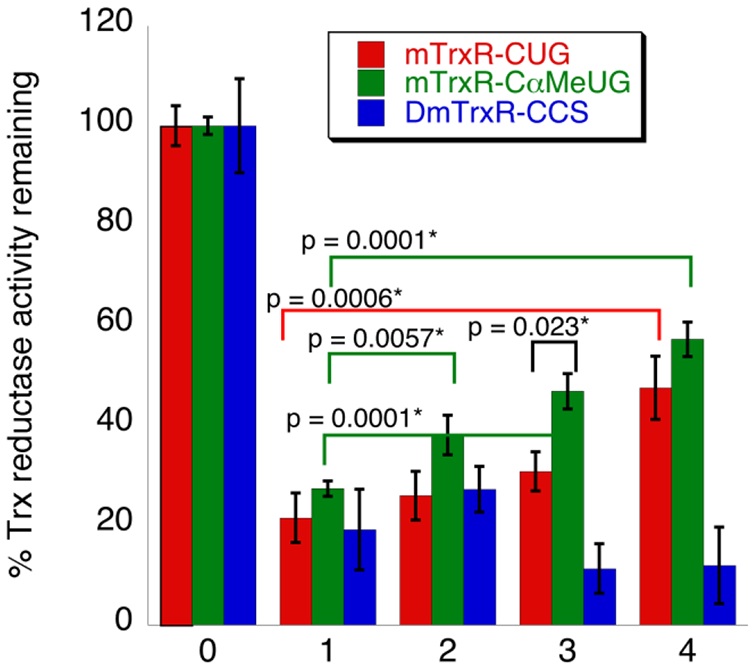
For all, Trx reductase activity was monitored by depletion of NADPH spectrophotometrically at A340. mTrxR-CUG (red), mTrxR-C(αMe)UG (green), and DmTrxR-CCS (blue) were inhibited by acrolein (condition 1), and no reversal reaction was attempted for this condition. Next, acrolein adduction was reversed via one of the following: (condition 2) 5 mM imidazole; (condition 3) 2 mM H2O2; or (condition 4) 2 mM H2O2 and 5 mM imidazole. All “reversal” reactions were conducted in 200 mM potassium phosphate, 1 mM EDTA, pH 7, at 37 °C for 10 min. Condition 0 (control) represents the normalized Trx reductase activity of all enzymes before acrolein inhibition or reversal, and all other bars represent the amount of activity remaining relative to the acrolein-untreated control. The calculated p-values (see Methods) between relevant sets of data are indicated in the figure and the * indicates significance at the 95% confidence level.
