Abstract
Background
Tobacco, alcohol and opioid misuse are associated with substantial morbidity and mortality among people with HIV (PWH). Despite existence of evidence-based counseling and medications for addiction, these treatments are infrequently offered in HIV clinics. The Working with HIV clinics to adopt Addiction Treatment using Implementation Facilitation (WHAT-IF?) study was conducted to address this implementation challenge. The study's goals were to conduct a formative evaluation of barriers to and facilitators of implementing addiction treatment for PWH followed by an evaluation of the impact of Implementation Facilitation (IF) on promoting adoption of addiction treatments and clinical outcomes.
Methods
The study was conducted at four HIV clinics in the northeast United States, using a hybrid type 3 effectiveness-implementation stepped wedge design and guided by the Promoting Action on Research Implementation in Health Services Research (PARiHS) framework. A mixed-methods approach was used to identify evidence, context, and facilitation-related barriers to and facilitators of integration of addiction treatments into HIV clinics and to help tailor IF for each clinic. An evaluation was then conducted of the impact of IF on implementation outcomes, including provision of addiction treatment (primary outcome), organizational and clinician and staff readiness to adopt addiction treatment, and changes in organizational models of care used to deliver addiction treatment. The evaluation also included IF's impact on effectiveness outcomes, specifically HIV-related outcomes among patients eligible for addiction treatment.
Conclusions
Results will generate important information regarding the impact of IF as a reproducible strategy to promote addiction treatment in HIV clinics.
Keywords: HIV, Substance-related disorders, Hybrid design, Implementation science
Highlights
-
•
Adoption of effective addiction treatments is inconsistent in HIV clinics.
-
•
Implementation Facilitation (IF) may help promote addiction treatments in HIV clinics.
-
•
Hybrid and stepped wedge designs are useful for evaluating the impact of IF.
-
•
We describe the design of an evaluation of IF for addiction treatment in HIV clinics.
1. Introduction
Globally, substance use disorders are common and contribute to significant morbidity and mortality among people with HIV (PWH) [[1], [2], [3]]. Tobacco use disorder leads to more years of life lost than HIV, particularly among those who are receiving antiretroviral treatment [4]. Alcohol use disorder contributes to worse outcomes along the HIV care continuum and is independently associated with increased risk of liver disease, cardiovascular disease, and malignancies and sexual risk behaviors with ongoing HIV transmission [3]. Similarly, opioid use disorder is associated with poorer engagement in HIV care and viral suppression, overdose death, and risk behaviors [[5], [6], [7]]. Fortunately, effective evidence-based counseling and medication treatments (referred to as “addiction treatments”) for each of these conditions are available, can be safely provided to PWH receiving antiretroviral therapy (Table 1 ) [[8], [9], [10], [11], [12], [13], [14]], and are recommended by clinical guidelines [15]. Importantly, patient health outcomes are better when addiction and HIV care are delivered in an integrated fashion [14,16,17].
Table 1.
Evidence-based counseling and medication treatments by substance.
| Substance | Counseling [72] | Medication [15] |
|---|---|---|
| Tobacco | -Brief intervention | -Nicotine replacement therapy -Bupropion -Varenicline |
| Alcohol | -Brief intervention (not for alcohol use disorder) -Cognitive behavioral therapy -Motivational enhancement therapy |
-Disulfiram -Acamprosate -Naltrexone (oral, injectable) |
| Opioid | -Cognitive behavioral therapy -Contingency management -Motivational enhancement therapy -Drug counseling |
-Methadone -Buprenorphine -Naltrexone (injectable) |
To date, routine adoption of addiction treatments in HIV clinics has been inconsistent [[18], [19], [20], [21]]. This is due in part to patients who are ambivalent about substance use treatment [22], but it is also a function of some clinicians who are hesitant to adopt addiction treatments because they have limited knowledge of and discomfort with these evidence-based practices [23,24]. To address this urgent implementation gap, innovative, feasible, reproducible strategies that promote sustainable adoption are needed. Implementation Facilitation (IF), also known as “Practice Facilitation,” may represent one solution [25,26]. Endorsed by the Agency for Healthcare Research and Quality [27], IF is defined as a “a multi-faceted process of enabling and supporting individuals, groups and organizations in their efforts to adopt and incorporate clinical innovations into routine practices.” The literature suggest that IF is an effective implementation strategy for promoting evidence-based practices that address chronic diseases [25,26]. Whereas IF is actively being applied to promote specific addiction treatments in general medicine settings [28,29], to our knowledge, it is has not yet been applied to promote addiction treatment in HIV clinics and with a simultaneous focus on three distinct substances. A strength of the IF approach is the inclusion of a formative evaluation. Defined as “a rigorous assessment process designed to identify potential and actual influences on the progress and effectiveness of implementation efforts,” [30] the formative evaluation allows for tailoring and adaptation of IF activities to meet site-specific needs and acknowledges the role of contextual factors in promoting implementation of evidence-based practices. This may be particularly relevant for integration of addiction treatment into HIV clinics, where a variety of factors such as the substance, clinician expertise, and resources, may impact the best model for such care integration. For example, all clinicians may be interested and willing to provide addiction treatment on site to each of their patients; prefer to have a clinician who is already part of the clinical team serve as the “specialist”, or decide that new expertise need to be brought into the clinic or that all patients should be referred elsewhere [31]. Notably, preferences may vary across substance and clinicians within a particular site or across sites and different team members may be involved [32].
Given this, we conducted the Working with HIV clinics to adopt Addiction Treatment using Implementation Facilitation (WHAT-IF?) study. Employing a hybrid type 3 effectiveness-implementation stepped wedge design [33,34], the goals of this study were to first conduct a formative evaluation of barriers to and facilitators of implementation of addiction treatment grounded in the Promoting Action on Research Implementation in Health Services (PARiHS) framework [35]. Defined as a “rigorous assessment process designed to identify potential and actual influence on the progress and effectiveness of implementation efforts,” formative evaluation can be done at several stages during an implementation effort. For instance, pre-implementation, the formative evaluation can be directly used to inform selection and tailoring of the site specific implementation efforts [30]. Our formative evaluation was guided by PARiHS, a widely applied framework that was specifically developed to understand the factors impacting implementation of evidence-based practices in clinical settings [35,36]. Then, we evaluated the impact of IF on promoting adoption of addiction treatments (primary outcome); clinician and staff as well as organizational readiness to provide addiction treatment; a description of models for delivering addiction treatment; and clinical outcomes. Herein, we describe the rationale, aims, and study design of the WHAT-IF? study.
2. Methods
2.1. Overall design
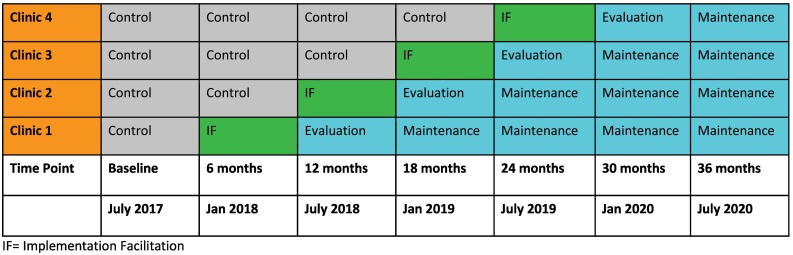
Funded by the National Institute on Drug Abuse (NIDA) as part of a dedicated initiative to promote integration of infectious diseases and substance use intervention services for individuals with HIV [37], WHAT-IF? was a multi-site hybrid type 3 effectiveness-implementation study that used a stepped wedge design to randomly assign sites to the onset of study activities (i.e., IF) (Fig. 1 ) [33,34]. Consistent with a hybrid type 3 effectiveness-implementation design where evaluation emphasis is on delivery of the treatment rather than patient outcomes [34], the primary outcome for this study was provision of addiction treatment, assessed using electronic health record (EHR) data upon completion of IF. Secondary outcomes included additional implementation outcomes (clinician and staff as well as organizational readiness to provide addiction treatments; models of care) and patient-level effectiveness outcomes (antiretroviral regimen receipt; HIV viral suppression; VACS Index 2.0 scores; and retention in HIV care).
Fig. 1.
WHAT-IF? Study Timeline with stepped wedge design.
IF = Implementation facilitation.
*Clinician and staff surveys were collected every 6 months prior to start of new period. The initial site visit, including qualitative data collection as part of the formative evaluation, were the first set of activities completed when a site rolled into the IF period.
2.2. Rationale for study design
We focused on promoting addiction treatment to address tobacco, alcohol, and opioid use given their prevalence and adverse impact on individuals with HIV and the existence of evidence-based counseling- as well as medication-based treatment options to address use of these substances that are recommended by clinical guidelines and can be provided in HIV clinics.
A hybrid type 3 effectiveness-implementation approach was appropriate given the existing evidence and clinical guidance supporting the use of addiction treatment among PWH and our interest in primarily evaluating the impact of the implementation strategy on practice change. In a stepped wedge design, a variation of a cluster randomized trial, clusters (namely, clinics in this study) are randomly assigned to the time at which they receive the intervention [33,[38], [39], [40]]. This approach was chosen for this study as it: 1) allows all sites to receive the intervention, which is important when there is lack of clinical equipoise and it is desirable for all study units to receive the intervention; 2) facilitates conduct of the study related to logistical and personnel challenges with a small team of investigators leading the IF; and 3) allows for consideration of temporal trends, which is particularly relevant given the heightened focus on the opioid epidemic [33].
We chose IF as the implementation strategy for several reasons. First, it is an effective implementation strategy (typically including audit and feedback; goal setting; system-level change; and collaborative meetings) for promoting evidence-based practices to address chronic diseases in clinical settings [25,26]. To our knowledge, however, only few studies have applied IF or its components to promoting adoption of substance use disorder treatment [29,[41], [42], [43]] and in a pilot fashion [44]. Second, given limited clinician training and potentially stigmatizing attitudes associated with addiction and existence of systems which have traditionally siloed addiction treatment into specialty settings outside of routine medical care, we believed stakeholder engagement would be particularly important. A strength of IF is that stakeholder engagement, including through the conduct of the formative evaluation, is inherent to the approach. Third, IF allows for flexibility such that different approaches to promote delivery of a given evidence-based practice may be adapted; given the variable contexts of our participating sites and desire to enhance generalizability to a range of settings, IF was deemed an appropriate implementation strategy. Fourth, we believed that a multi-pronged approach would be required that included more intensive engagement during the IF phase, that then tapered during evaluation and maintenance phases with the ongoing Learning Collaborative. Last, members of our team had experience with IF components [32,[45], [46], [47]]. To guide our approach, we chose the PARiHS framework, which has identified three core elements for determining whether an evidence-based practice will be successfully implemented into clinical care: 1) the nature of the evidence (e.g., research, clinical experience) and key stakeholder's perceptions of that evidence; 2) qualities of the context in which the evidence is being introduced and enacted upon; and 3) the facilitation (i.e., implementation intervention), the strategy used to make it easier for both individuals and an organization to adopt a practice [35,36]. It is therefore a usual framework for guiding qualitative work to understand barriers and facilitators to implementation of a given evidence-based practice [35] and also has been applied to guide the development of the Organizational Readiness to Change Assessment (ORCA), a quantitative assessment of organizational readiness to adopt a given evidence-based practice [48].
2.3. Study context, coordinating center, and institutional review
The study was conducted in the context of the Yale's Center for Interdisciplinary Research on AIDS (CIRA)-supported New England HIV Implementation Science Network, whose mission includes stimulating and supporting research and evaluation collaborations across New England and promoting implementation science in small urban areas with a high prevalence of HIV [49]. The coordinating center is located at Yale School of Medicine, in New Haven, CT and the Yale Center for Analytical Sciences (YCAS) coordinates data management and analytic support. The four participating sites included: 1) Haelen Center at Yale-New Haven Hospital, New Haven, CT; 2) the Community Care Center at Hartford Hospital's HIV Clinic, Hartford, CT; 3) the Miriam Hospital Immunology Center, the Miriam Hospital, Providence, RI; and 4) the STAR Health Center, SUNY Downstate Medical Center, Brooklyn, NY. These four sites are all based in urban settings, but vary in terms of their affiliations (e.g., academic vs. community-based hospital clinic), infrastructure (e.g., on-site behavioral health), and resources (e.g., external grant funding). All sites rely on an EHR. The study was approved by the Institutional Review Boards at Yale University and at each of the participating universities and healthcare sites.
2.4. Study participants
The study included two distinct participant groups who contributed both quantitative and qualitative data to the study: 1) clinicians and staff and 2) PWH actively receiving care at the participating clinics.
2.4.1. Clinicians and staff participants
All clinicians, including prescribing (i.e., physicians, nurse practitioners, physician assistants) and non-prescribing clinicians (e.g., psychologists, social workers), as well as staff (e.g., nurses, community health workers) who had been employed at the given site for at least six months, were invited to participate in qualitative and quantitative data collection. We purposefully sought to elicit perspectives of diverse clinicians and staff given that 1) team-based multidisciplinary care is the norm in HIV clinics, 2) perspectives across clinicians and staff regarding addiction treatment could impact implementation of that treatment, and 3) different disciplines could serve a role in optimizing addiction treatment depending on resources and the context. Clinicians and staff were provided a meal and received a $50 gift card for participation in a focus group. In addition, meals and/or restaurant gift cards were offered at some sites to incentivize survey completion.
2.4.2. Patient participants
At each of the four participating clinics, a sample of PWH who had a diagnosis of a tobacco, alcohol and/or opioid use disorder were invited to participate in a focus group. We purposefully sampled individuals who had and had not received effective treatment to address their addiction within the HIV clinic; patients were identified and recruited for participation based on their involvement in ongoing support groups and/or based on research team awareness of the patients by direct clinical experience or referral by other clinical staff. Patients were provided a meal and received a $25 gift card for participation in a focus group.
In addition, we used EHR data on all PWH actively receiving care in the participating clinics from July 16, 2016 through July 25, 2020 who had been diagnosed with a tobacco, alcohol and/or opioid use disorder. Patients were identified as having HIV using the visit reason and international diagnostic codes (ICD-9 and ICD-10). Patients were considered to be actively receiving care at a participating site if they had a scheduled visit at the clinic during the time period of interest, regardless of whether they attended the visit. Patients were eligible to enter the cohort (i.e., open cohort design) at any point during the study period once they met inclusion criteria. We intended to be inclusive in considering a patient active in care given that patients may cycle in and out of care [50,51].
2.5. Randomization
Due to the potential for contamination from a distinct NIDA-funded project at The Miriam Hospital Immunology Center, this site was assigned to receive IF last. However, that project was never initiated, thereby reducing concerns regarding potential contamination. The other three study clinics were randomized to the time at which they would begin IF by the YCAS. Members of the investigative team and study sites remained blinded to the sequence until approximately 6 weeks prior to the start of IF to allow for planning of site visits.
2.6. Study assessments
The PARiHS framework [35,36] guided the quantitative and qualitative assessments. Specifically, we assessed ratings and perspectives on the evidence for addiction treatments (“evidence”), the HIV clinical context for delivering such treatments (“context”), and support needs and efforts for promoting adoption of addiction treatments in HIV clinics (“facilitation”).
2.6.1. Site description survey
At each site, a clinic director or medical director completed a Qualtrics™ survey at survey initiation and then every six months over the course of the 3.5 years for a total of seven surveys to provide information on their patients served and resources, particularly as it related to provision of addiction treatment. Survey items included assessed clinic volume, patient demographics and insurance status, estimated prevalence of substance use disorders, HIV viral suppression, and retention in care. In addition, we collected data on types and availability of different clinical expertise available in the clinic (e.g., addiction medicine, addiction psychiatry, clinicians certified to prescribe buprenorphine, social workers); availability of on-site and off-site treatments for tobacco, alcohol, and opioid use disorder (e.g., counseling, case management, harm reduction, addiction specialist, outreach services); associated processes for linking patients to treatment (e.g., patient education about community resources, written referral, appointment, navigation); and current model for delivering care for tobacco, alcohol and opioid use disorder (Appendix 1). For example, to assess the clinic's approach to providing treatment for opioid use disorder, the survey asks: “How would you describe your clinic's current approach to providing treatment for opioid use disorder?” where options include: 1. “Each clinician treats both HIV and opioid use disorder in their own patient panel;” 2. “At least one clinician in our clinic provides onsite treatment of opioid use disorder for their own patients and other clinicians' patients,” 3. “Specialists provide treatment of opioid use disorder onsite at our clinic, but do not provide HIV primary care for any patients,” and 4. “Specialists provide treatment for opioid use disorder outside of our HIV clinic (e.g. by referral).” These options are intended to capture the spectrum of possible care integration, including on the clinician level, clinic level, or system level. We additionally assess availability of on-site and off-site services to address each substance (e.g., substance use counseling, case management, addiction specialist, outreach services) and referral mechanism to off-site services.
2.6.2. Clinician and staff survey
After being provided an introduction to the survey from their medical director, clinicians and staff were invited to complete a confidential, web-based survey collected via Qualtrics™ at the same time points as the site description survey (i.e., study initiation and every six months for a total of seven surveys). Surveys are open for six weeks with weekly reminders. Surveys were timed to occur at the end of each 6-month period (Fig. 1) and immediately prior to IF-related activities commencing at a site newly starting the intervention. Prior to filling out the survey, individuals were provided information about the survey and its purpose; the decision to complete the survey was considered consent to study participation. Based on our prior experiences [24,31,52,53], literature review, and initial pilot testing and refinement with our multidisciplinary team, the survey was developed and designed to assess: 1) demographics and expertise; 2) past 6-month and lifetime experiences with prescribing and/or referring patients for counseling and medications to address tobacco, alcohol and opioid use; 3) preferred model of care for addressing tobacco, alcohol and opioid use (e.g. all clinicians are trained to deliver treatment, on-site specialist is available for referral) [31]; 4) readiness to provide or refer patients for treatment assessed by a readiness ruler (where response anchors included 0 = not ready, 10 = ready and responses were dichotomized as 0- < 7 = least ready; ≥7–10 = most ready) [29]; and 5) ratings on the ORCA, which was adapted from the original measure [48] to focus on adoption of counseling and medications to address tobacco, alcohol, and opioid use in HIV clinics (Appendix 1). Grounded in the PARiHS framework, the ORCA has been used to evaluate and predict the impact of implementation interventions [54,55] and was used by our team in an implementation study designed to promote buprenorphine initiation in Emergency Departments [29]. Participants were asked to rate 1) the “evidence” supporting each evidence-based practice, 2) the HIV clinic “context” as a setting for delivering addiction treatments, and 3) the “facilitation” efforts following IF initiation. For example, for the Evidence subscale regarding tobacco treatment medications, we asked: “In my opinion prescribing of medications in my clinic to decrease smoking will improve health outcomes among patients who smoke cigarettes;” response options included a 5-point Likert scale (1-strongly disagree, 2-disagree, 3-neither agree nor disagree, 4-agree, 5-strongly agree). The Context scale contains five subscales: Leadership Culture; Staff Culture; Leadership Practice; Evaluation Accountability; and Opinion Leader Culture. An additional item assesses Slack Resources to examine resources available to support practice change. Subscale response options also included “don't know” or “not applicable,” which were recoded as “neither agree nor disagree” or “neither frequently nor infrequently” to allow computation of subscale scores [56].
2.6.3. Stakeholder focus groups
Upon IF initiation at each site and to gain a deep understanding of and contextualize the quantitative data, we conducted two focus groups at each site including one with clinicians and staff and a second with patients. The goals of these focus groups were to understand the: 1) degree of implementation of addiction treatment; 2) determinants of the current practices; 3) potential barriers to and facilitators of practice change; and 4) feasibility of the planned WHAT-IF? IF activities [30]. Focus groups were led by physicians with expertise in internal medicine, psychiatry, addiction medicine, HIV, qualitative methods and implementation science and grand tour questions were informed by the PARiHS framework (Appendix 2). Participants also completed a brief demographic survey.
These focus groups were digitally recorded and transcribed. We used a rapid assessment process [57,58] to inform IF activities and summarize and share findings with the medical director. We then followed this by a more detailed directed content analysis [59], whereby members of the investigative team independently reviewed each of the transcripts line by line to develop and refine the codebook and reach consensus on codes and thematic saturation [60]. After there was consensus on the codebook and assigned codes, one investigator reviewed all transcripts to confirm these codes were consistently applied to all transcripts and entered into NVivo software to facilitate data organization and retrieval. Themes were then generated based on coded quotations and discussion with the research team within the PARiHS framework.
2.6.4. Electronic health record data
Among active PWH, we extracted past 12-month data at baseline and then in 6-month intervals during the study period on demographics, diagnoses, receipt of counseling and medications, visit frequency, and Veterans Aging Cohort Study (VACS) Index 2.0 score [61] components based on data collected as part of routine clinical care. Demographics variables included: age, race, ethnicity, gender, first three digits of zip code (as a proxy for socioeconomic status and to keep data de-identified), and insurance status. Diagnostic variables included presence of substance use disorders and mental illness based on the problem list, encounter reason and ICD-9 and ICD-10 codes [62]. Counseling to address substance use was captured based on encounters with a clinician, social worker, or psychologist and included psychiatric and substance use assessments, individual and group psychotherapy, individual counseling, case management, crisis intervention, prolonged services, family services, and health and behavior education [63]. Medications included prescription for antiretroviral agents consistent with an active HIV treatment regimen [64] and medications to treat addiction (Table 1) that may be provided through HIV clinics. We assumed medications were taken as prescribed and assessed medication coverage in a given 6-month interval by determining days supplied with a given prescription such that a single prescription could span two separate intervals. For injectable naltrexone, we assumed the medication was active for 30 days, and administered on schedule as prescribed. Visits included completed office and follow-up visits to the HIV clinic with a clinician. Health status measures, which were ascertained based on labs closest to baseline and the end of each 6-month interval, included HIV biomarkers (CD4 cell count, HIV viral load) and the VACS Index 2.0 score [61]. The VACS Index 2.0 scores is calculated based on HIV biomarkers and additionally white blood cell count, hemoglobin, aspartate aminotransferase, alanine aminotransferase, serum creatinine, hepatitis C virus (HCV) antibody and RNA, albumin and body mass index [61]. The VACS Index score is a validated measure of morbidity and mortality that is sensitive to changes in substance use and its treatment among PWH [65,66].
2.7. Implementation intervention: Implementation facilitation
For the current study, we adapted an existing IF manual used to promote mental health treatment into primary care [67] (Appendix 3), which we have since further adapted for promoting buprenorphine initiation in Emergency Departments [29]. IF included a bundle of activities designed to promote addiction treatment into HIV clinics tailored to site specific needs (Table 2 ). Informed by the survey data collected prior to IF initiation and information regarding the clinical organizational structure, IF activities began with a formative evaluation of barriers and facilitators to promoting addiction treatments in HIV clinics. This involved a full day site visit to each site during which initial focus groups were conducted with stakeholders, as well as a face-to-face meeting with the medical director, tour of the HIV clinic, review of workflow and electronic medical record documentation. These initial site visits were followed by two follow-up visits to each site during which academic detailing was conducted and efforts were made to bridge silos across disciplines (e.g., Addiction Psychiatry and/or Addiction Medicine and Infectious Disease) within the same institution. These visits were complemented by ongoing communications (via email, telephone) with the sites to facilitate additional IF activities. In addition, upon initiation of the IF period, sites were invited to join the Learning Collaborative activities, which extended for the duration of the study to support implementation efforts. Throughout the course of the study, we tracked conduct and participation in IF activities using a tracking log [68]. This included, for example, how many clinicians and staff received academic detailing; delivery of on-site lectures and journal clubs focused on addiction treatment; and timing and content of Learning Collaborative calls.
Table 2.
| Component | General description | Specifics for WHAT-IF? |
|---|---|---|
| External facilitator | Outside content and implementation expert(s) who assists site | Facilitators included members of the investigative team with expertise in internal medicine, addiction medicine, addiction psychiatry, HIV and implementation science; led all aspects of IF activities in collaboration with sites |
| Formative evaluation | Quantitative and qualitative determination of potential and actual influences on progress and effectiveness of implementation efforts [30] | Guided by the PARiHS framework [35], web-based survey of clinicians and staff followed by site visits that included: focus groups with stakeholders (including patients), face to face meeting with medical directors, HIV clinic tour, review of patient flow and electronic health record documentation, discussion of existing quality improvement (QI) practices, meeting with potential local champions at IF onset. Two follow-up site visits and additional communication by email, telephone, videoconference. |
| Local champion | Local site stakeholder(s) who promotes change | Self-identified individual with expertise and/or interest in promoting practice change to address tobacco, alcohol, and/or opioid use; may have experience in QI and/or be the medical director. Becomes point-person(s) for external facilitators. |
| Education with academic detailing (AD) | Provision of unbiased peer education | After a subset of investigators participated in AD training [73], AD pamphlets were created and AD was performed with front-line clinicians and staff during site visits conducted during IF. Information regarding X-waiver training opportunities, professional conferences, relevant journal articles, and clinical guidelines additionally distributed by external facilitators. Efforts made to facilitate Grand Rounds and pre-clinic conferences focused on addressing addiction in HIV with local experts and to facilitate intra-institutional collaborations for clinical consultation, shadowing opportunities (e.g., observing buprenorphine treatment initiation). |
| Stakeholder engagement | Aligning goals of implementation and those impacted | Initial site visits serve to assess interest and perceived relevance in promoting addiction treatment in HIV clinics; based on the initial formative evaluation, the external facilitators share feedback with HIV medical director organized by PARiHS framework to propose a comprehensive approach for stimulating practice change. |
| Tailoring program to site | Addressing site specific needs based on formative evaluation, problem identification and resolution, assistance with technical issues | Based on local resources and expertise, external facilitators work with local champion to implement most feasible model for enhancing delivery of tobacco, alcohol, and opioid use treatment. |
| Performance monitoring and feedback | Assess implementation of screening and treatment efforts and inform sites of results | Provision of electronic health record-based data demonstrating prevalence of diagnoses of tobacco, alcohol and opioid use disorder and proportion receiving treatment shared with medical director; in addition, ½ day site visit performed to assess performance on care integration as measured by the opioid use disorder and HIV integration (OHI) index [74]. |
| Establishing a learning collaborative | Shared learning opportunities tailored to stakeholders | Monthly videoconference hosted by WHAT-IF? team to facilitate mutual learning and clinic updates, included a mixed of didactics and case-based discussion. Monthly newsletter to disseminate upcoming learning opportunities (e.g., trainings and conferences), newly published peer-reviewed articles and guidelines. |
| Program marketing | Efforts designed to increase attention to availability of on-site and addiction treatment services | Pins, pads, pens, buttons and posters with “WHAT-IF?” logo created and shared with clinic teams to help increase awareness of the project and facilitate patient-clinician discussions (“WHAT-IF? what…”) |
2.8. Statistical considerations
2.8.1. Sample size calculations
Consistent with the goals of a hybrid 3 effectiveness-implementation study, the primary goal of this study was to assess the impact of IF on provision of addiction treatment among eligible patients by the clinics over time (baseline vs. evaluation vs. maintenance periods). Informed by prior work, we anticipated an 11% absolute increase during the evaluation phase and 19% absolute increase during the maintenance phase from baseline. A parallel group design, unadjusted for clustering and repeated measures would require a sample size of 592 (296 in each the control and intervention arms) to detect the estimated effect size assuming 90% power and Type I error of 0.05. To account for the stepped wedge design, we made the following assumptions: 1) each clinic would provide a minimum of 300 addiction treatment eligible patients; 2) intracluster correlation, ρ = 0.001; 3) number of steps, κ = 4; 4) number of baseline measurements, b = 1; and 5) number of measurements taken after each step, t = 1 [69]. This yielded a derived design effect of 0.63 and assuming a cross-sectional design, a required adjusted sample size of 375 across the four clinics.
2.8.2. Statistical analyses
2.8.2.1. Primary (implementation) outcome
The primary implementation outcome for this study was the change in the percentage of treatment eligible patients who received addiction treatment during the evaluation and maintenance periods compared to the baseline period.
2.8.2.2. Secondary implementation outcomes
Secondary implementation outcomes included ORCA and readiness rulers scores, each as continuous outcomes, and models of care used to deliver addiction treatment.
2.8.2.3. Effectiveness outcomes
We additionally evaluated the impact of IF on effectiveness (i.e., patient-level outcomes) among patients diagnosed with a tobacco, alcohol or opioid use disorder. Secondary outcomes included: prescription for an active antiretroviral regimen; HIV viral suppression, defined as an HIV RNA <200 copies/mL15; VACS Index 2.0 score; and retention in HIV care, assessed by whether patients had at least one visit in a 6-month period [70].
2.8.2.4. Statistical analysis
Characteristics of patients and clinics will be shown by randomization status in each step of the design. For all analyses, we will use an intent-to-treat approach according to the time clinics were intended to cross over from control condition to IF. For the primary implementation outcome, we will use a generalized linear mixed model, adjusting for calendar time (a potential confounder due to its association with both exposure to the intervention and outcome), with a random effect for clinic, a fixed effect for each step and allowing for repeated measures for patients in the clinics. Since the population of the clinics participating in this study are generally stable, rendering this a cohort stepped wedge design, an additional random effect for patients in each clinic will be included. We will apply a similar approach to evaluate the impact of IF on ORCA subscale scores and readiness ruler scores. Additional analyses will be used to evaluate whether ORCA subscale scores mediated the proportion of treatment eligible patients receiving addiction treatment [71].
To determine the impact of IF on models of care, we will describe the extent to which provision of treatment for each substance is coordinated (facilitated by the clinic), co-located (provided in the clinic) or integrated (provided by the primary HIV clinician) by clinic. We will track changes in these models of care from the control period to the evaluation and maintenance periods.
We will examine each of the HIV-related secondary effectiveness outcomes at the 6- month intervals of control, evaluation and maintenance phases based on the provision of addiction treatment. We will determine the association between provision of addiction treatment and effectiveness outcomes using the repeated measures MIXED models for continuous outcomes and generalized estimating equations for dichotomous outcomes. We will adjust for demographics, substance use disorder and psychiatric diagnoses. We will describe the associations with least squares means or odds ratios and 95% confidence intervals.
2.9. Current status
As of June 30, 2020, all sites are in the “maintenance phase” of the study. To date, there have been several unpredictable factors impacting study implementation. First, as above, due to a competing NIDA-funded study at one of the sites, we intentionally assigned this site to receive IF last to avoid contamination. Second, study timing overlapped with changes in the EHR; at one site, this occurred in the beginning of the study while in a second site, this occurred during the last period of the study with potential impact on how well diagnoses are captured. This may impact denominators (i.e., patients identified as eligible for addiction treatment) due to changes in charting practices and numerators (given the impact of EHR changes on clinical practice during the transition phase). Third, the novel coronavirus (COVID-19) resulted in a global pandemic beginning March 2020 in the northeast United States. This has yielded substantial changes in the healthcare system, including decreased on-site clinic visits and the use of telehealth for routine HIV care. Since this event occurred toward the end of our study, we believe this threat to the validity of our findings will be minimal; however, we will conduct sensitivity analyses excluding the data that overlap with this time period. As of September 8, 2020, sites are finalizing extractions from the EHR for the final study period and efforts to disseminate findings (i.e., abstract submissions, manuscripts) are anticipated starting in 2021.
3. Discussion
Strategies are needed to increase the provision of addiction treatment to address tobacco, alcohol and opioid misuse in HIV clinics. This protocol describes a novel application of IF to enhance delivering of treatments for unhealthy substance use for which there are both counseling and medication-based options that are suitable for delivery in HIV clinics. In addition, by using a hybrid type 3 effectiveness-implementation design, we will gain critical data regarding both the impact of IF on the clinic, clinician and staff, as well as on patients. Given the variability in the clinic structures, we will learn important lessons for implementation of addiction treatment in both academic and community-based settings.
3.1. Limitations
There are several limitations to our protocol. First, we will be using electronic health record to assess adoption of counseling services to address opioid, alcohol, and tobacco use disorder, but will be limited in our ability to confirm that substance use was specifically addressed during these sessions. In secondary analyses, we will be able to evaluate the concordance between electronic medical record data and self-reported provision of counseling among clinicians and staff using the survey data. Second, while we focus on provision of addiction treatment as a primary outcome of this study; we did not evaluate how IF translates into quality of care. Future studies of IF and other implementation strategies, however, should focus on assessment of the quality of care that is provided. Third, findings from this study may not be generalizable to clinics located in other parts of the United States. Fourth, we may not be able to fully account for factors that may lead to disruptions in clinical care (e.g., EHR transition, clinic moves) and staffing changes. Fifth, based on our measurement of adoption, we will be unable to determine whether any observed gaps in provision of addiction treatment are driven by clinician or patient level factors. Lastly, given variable practices in screening for and documenting presence of substance use and substance use disorders, the denominators will likely be an underestimate of true prevalence.
3.2. Conclusion
The current study will provide much needed data on the impact of a reproducible, adaptable strategy for promoting treatment of tobacco, alcohol, and opioid misuse in HIV clinics. Given the profound individual and public health impacts of these conditions among PWH, solutions for promoting adoption of guideline-recommended care are urgently needed.
Disclosures
Earlier versions of this work were presented at the Addiction Health Services Research Conference, Seattle, Washington, 2017, the 11th Annual Conference on the Science of Dissemination and Implementation in Health, Washington, D.C., 2018, and the College on Problems of Drug Dependence 2020 Scientific Meeting, Virtual Conference, 2020.
Role of the funding source
The investigators had primary responsibility for study design, data collection, data analysis, data interpretation, and writing of the manuscript. The National Institute on Drug Abuse did not contribute to design of data collection, data analyses, data interpretation or decision to submit this work for publication.
Funding
This work was funded by the National Institute on Drug Abuse (R01DA041067) and partially supported through Yale's CTSA Award (UL1TR001863). EJ Edelman received funding from the NIDA-funded Yale-Drug Use, Addiction and HIV Research Scholars Program (grant #K12DA033312) during the conduct of this work.
Declaration of Competing Interest
SM reported participating in an advisory board meeting of Alkermes in the past year. The authors have no other conflicts of interest to disclose.
Acknowledgements
EJE, JD, DE, WCB, and DAF contributed to the design of the study and obtaining grant funding. EJE, EP, JD, DE, and DAF oversaw conduct of the study. EJE, KM, SBM, and DAF participated in implementation of the intervention. PAC, DHC, GR, JY oversaw study implementation at participating sites. EJE and EP contributed to the initial draft of the manuscript. All authors contributed to critical revision of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2020.106156.
Appendix A. Supplementary data
WHAT-IF? Provider and Staff Survey (baseline)
WHAT-IF? Focus group guides
WHAT-IF? Implementation Facilitation Manual
References
- 1.Hartzler B., Dombrowski J.C., Crane H.M. Prevalence and predictors of substance use disorders among HIV care enrollees in the United States. AIDS Behav. 2017;21(4):1138–1148. doi: 10.1007/s10461-016-1584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mdege N.D., Shah S., Ayo-Yusuf O.A., Hakim J., Siddiqi K. Tobacco use among people living with HIV: analysis of data from demographic and health surveys from 28 low-income and middle-income countries. Lancet Glob. Health. 2017;5(6):e578–e592. doi: 10.1016/S2214-109X(17)30170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams E.C., Hahn J.A., Saitz R., Bryant K., Lira M.C., Samet J.H. Alcohol use and human immunodeficiency virus (HIV) infection: current knowledge, implications, and future directions. Alcohol. Clin. Exp. Res. 2016;40(10):2056–2072. doi: 10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helleberg M., May M.T., Ingle S.M. Smoking and life expectancy among HIV-infected individuals on antiretroviral therapy in Europe and North America. AIDS. 2015;29(2):221–229. doi: 10.1097/QAD.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korthuis P.T., Fiellin D.A., McGinnis K.A. Unhealthy alcohol and illicit drug use are associated with decreased quality of HIV care. J. Acquir. Immune Defic. Syndr. 2012;61(2):171–178. doi: 10.1097/QAI.0b013e31826741aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosh N., Dong X., Lyss S., Mendoza M., Mitsch A.J. WA; Seattle: 2020. Opioid overdose deaths among persons with HIV infection, United States, 2011-2015, Abstract #147. Paper Presented at: CROI2019. [Google Scholar]
- 7.Edelman E.J., Chantarat T., Caffrey S. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug Alcohol Depend. 2014;139:79–85. doi: 10.1016/j.drugalcdep.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercie P., Arsandaux J., Katlama C. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 inter-ACTIV): a randomised controlled phase 3 clinical trial. Lancet HIV. 2018;5(3):e126–e135. doi: 10.1016/S2352-3018(18)30002-X. [DOI] [PubMed] [Google Scholar]
- 9.Pool E.R., Dogar O., Lindsay R.P., Weatherburn P., Siddiqi K. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst. Rev. 2016;6 doi: 10.1002/14651858.CD011120.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tetrault J.M., Tate J.P., KA McGinnis. Hepatic safety and antiretroviral effectiveness in HIV-infected patients receiving naltrexone. Alcohol. Clin. Exp. Res. 2012;36(2):318–324. doi: 10.1111/j.1530-0277.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelman E.J., Moore B.A., Holt S.R. Efficacy of extended-release naltrexone on HIV-related and drinking outcomes among HIV-positive patients: a randomized-controlled trial. AIDS Behav. 2018;23(1):211–221. doi: 10.1007/s10461-018-2241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altice F.L., Bruce R.D., Lucas G.M. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S22–S32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiellin D.A., Weiss L., Botsko M. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S33–S38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oldfield B.J., Munoz N., McGovern M.P. Integration of care for HIV and opioid use disorder: a systematic review of interventions in clinical and community-based settings. AIDS. 2018;33(5):873–884. doi: 10.1097/QAD.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health and Human Services . 2019. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. [Google Scholar]
- 16.BWRP Heath, Reynolds K. SAMHSA-HRSA. Center for Integrated Health Solutions; March 2013. A review and proposed standard framework for levels of integrated healthcare. [Google Scholar]
- 17.Walley A.Y., Palmisano J., Sorensen-Alawad A. Engagement and substance dependence in a primary care-based addiction treatment program for people infected with HIV and people at high-risk for HIV infection. J. Subst. Abus. Treat. 2015;59:59–66. doi: 10.1016/j.jsat.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Vijayaraghavan M., Yuan P., Gregorich S. Disparities in receipt of 5As for smoking cessation in diverse primary care and HIV clinics. Prev. Med. Rep. 2017;6:80–87. doi: 10.1016/j.pmedr.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oldfield B.J., McGinnis K.A., Edelman E.J. Predictors of initiation of and retention on medications for alcohol use disorder among people living with and without HIV. J. Subst. Abus. Treat. 2020;109:14–22. doi: 10.1016/j.jsat.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahrir S., Crothers K., KA McGinnis. Receipt and predictors of smoking cessation pharmacotherapy among veterans with and without HIV. Prog. Cardiovasc. Dis. 2020;63(2):118–124. doi: 10.1016/j.pcad.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyse J.J., Robbins J.L., McGinnis K.A. Predictors of timely opioid agonist treatment initiation among veterans with and without HIV. Drug Alcohol Depend. 2019;198:70–75. doi: 10.1016/j.drugalcdep.2019.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fredericksen R.J., Edwards T.C., Merlin J.S. Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care. 2015;27(10):1255–1264. doi: 10.1080/09540121.2015.1050983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chander G., Monroe A.K., Crane H.M. HIV primary care providers--screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016;161:59–66. doi: 10.1016/j.drugalcdep.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum P.J., Little S., Botsko M. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S91–S97. doi: 10.1097/QAI.0b013e31820a9a82. [DOI] [PubMed] [Google Scholar]
- 25.Wang A., Pollack T., Kadziel L.A. Impact of practice facilitation in primary care on chronic disease care processes and outcomes: a systematic review. J. Gen. Intern. Med. 2018;33(11):1968–1977. doi: 10.1007/s11606-018-4581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baskerville N.B., Liddy C., Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann. Fam. Med. 2012;10(1):63–74. doi: 10.1370/afm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.AHRQ Patient Centered Medical Home Resource Center. Practice Facilitation. https://pcmh.ahrq.gov/page/practice-facilitation (Accessed 3.10.2020)
- 28.AHRQ AHRQ Initiative To Reduce Unhealthy Alcohol Use. https://integrationacademy.ahrq.gov/about/opioids-substance-use/ahrq-alcohol-initiative (Accessed 3.10.2020)
- 29.D’Onofrio G., Edelman E.J., Hawk K.F. Implementation facilitation to promote emergency department-initiated buprenorphine for opioid use disorder: protocol for a hybrid type III effectiveness-implementation study (project ED HEALTH) Implement. Sci. 2019;14(1):48. doi: 10.1186/s13012-019-0891-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stetler C.B., Legro M.W., Wallace C.M. The role of formative evaluation in implementation research and the QUERI experience. J. Gen. Intern. Med. 2006;21(Suppl. 2):S1–S8. doi: 10.1111/j.1525-1497.2006.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelman E.J., Moore B.A., Calabrese S.K. Preferences for implementation of HIV pre-exposure prophylaxis (PrEP): results from a survey of primary care providers. Prev. Med. Rep. 2020;17:101012. doi: 10.1016/j.pmedr.2019.101012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss L., Netherland J., Egan J.E. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J. Acquir. Immune Defic. Syndr. 2011;56(Suppl. 1):S68–S75. doi: 10.1097/QAI.0b013e31820a8226. [DOI] [PubMed] [Google Scholar]
- 33.Mdege N.D., Man M.S., Taylor Nee Brown C.A., Torgerson D.J. Systematic review of stepped wedge cluster randomized trials shows that design is particularly used to evaluate interventions during routine implementation. J. Clin. Epidemiol. 2011;64(9):936–948. doi: 10.1016/j.jclinepi.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Curran G.M., Bauer M., Mittman B., Pyne J.M., Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med. Care. 2012;50(3):217–226. doi: 10.1097/MLR.0b013e3182408812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stetler C.B., Damschroder L.J., Helfrich C.D., Hagedorn H.J. A guide for applying a revised version of the PARIHS framework for implementation. Implement. Sci. 2011;6:99. doi: 10.1186/1748-5908-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helfrich C.D., Damschroder L.J., Hagedorn H.J. A critical synthesis of literature on the promoting action on research implementation in health services (PARIHS) framework. Implement. Sci. 2010;5:82. doi: 10.1186/1748-5908-5-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Institute on Health . 2015. Integration of Infectious Diseases and Substance Abuse Intervention Services for Individuals Living with HIV (R01) (RFA-DA-15-013) [Google Scholar]
- 38.Beard E., Lewis J.J., Copas A. Stepped wedge randomised controlled trials: systematic review of studies published between 2010 and 2014. Trials. 2015;16:353. doi: 10.1186/s13063-015-0839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown C.A., Lilford R.J. The stepped wedge trial design: a systematic review. BMC Med. Res. Methodol. 2006;6:54. doi: 10.1186/1471-2288-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hussey M.A., Hughes J.P. Design and analysis of stepped wedge cluster randomized trials. Contemp. Clin. Trials. 2007;28(2):182–191. doi: 10.1016/j.cct.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Hagedorn H., Kenny M., Gordon A.J. Advancing pharmacological treatments for opioid use disorder (ADaPT-OUD): protocol for testing a novel strategy to improve implementation of medication-assisted treatment for veterans with opioid use disorders in low-performing facilities. Addict. Sci. Clin. Pract. 2018;13(1):25. doi: 10.1186/s13722-018-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frost M.C., Ioannou G.N., Tsui J.I. Practice facilitation to implement alcohol-related care in veterans health administration liver clinics: a study protocol. Implement. Sci. Commun. 2020;1(1):68. doi: 10.1186/s43058-020-00062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon A.J., Drexler K., Hawkins E.J. Stepped care for opioid use disorder train the trainer (SCOUTT) initiative: expanding access to medication treatment for opioid use disorder within veterans health administration facilities. Subst. Abus. 2020;41(3):275–282. doi: 10.1080/08897077.2020.1787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris A.H.S., Brown R., Dawes M. Effects of a multifaceted implementation intervention to increase utilization of pharmacological treatments for alcohol use disorders in the US veterans health administration. J. Subst. Abus. Treat. 2017;82:107–112. doi: 10.1016/j.jsat.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Midboe A.M., Martino S., Krein S.L. Testing implementation facilitation of a primary care-based collaborative care clinical program using a hybrid type III interrupted time series design: a study protocol. Implement. Sci. 2018;13(1):145. doi: 10.1186/s13012-018-0838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker W.C., Mattocks K.M., Frank J.W. Mixed methods formative evaluation of a collaborative care program to decrease risky opioid prescribing and increase non-pharmacologic approaches to pain management. Addict. Behav. 2018;86:138–145. doi: 10.1016/j.addbeh.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Egan J.E., Casadonte P., Gartenmann T. The physician clinical support system-buprenorphine (PCSS-B): a novel project to expand/improve buprenorphine treatment. J. Gen. Intern. Med. 2010;25(9):936–941. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helfrich C.D., Li Y.F., Sharp N.D., Sales A.E. Organizational readiness to change assessment (ORCA): development of an instrument based on the promoting action on research in health services (PARIHS) framework. Implement. Sci. 2009;4:38. doi: 10.1186/1748-5908-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Center for Interdisciplinary Research on AIDS New England HIV Implementation Science Network. https://cira.yale.edu/network (Accessed 3.10.2020)
- 50.Edelman E.J., Tate J.P., Fiellin D.A. Impact of defined clinical population and missing data on temporal trends in HIV viral load estimation within a health care system. HIV Med. 2015;16(6):346–354. doi: 10.1111/hiv.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byrd K.K., Furtado M., Bush T., Gardner L. Reengagement in care after a gap in HIV care among a population of privately insured persons with HIV in the United States. AIDS Patient Care STDs. 2016;30(11):491–496. doi: 10.1089/apc.2016.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edelman E.J., Moore B.A., Calabrese S.K. Primary care Physicians’ willingness to prescribe HIV pre-exposure prophylaxis for people who inject drugs. AIDS Behav. 2017;21(4):1025–1033. doi: 10.1007/s10461-016-1612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackstock O.J., Moore B.A., Berkenblit G.V. A cross-sectional online survey of HIV pre-exposure prophylaxis adoption among primary care physicians. J. Gen. Intern. Med. 2017;32(1):62–70. doi: 10.1007/s11606-016-3903-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagedorn H.J., Heideman P.W. The relationship between baseline organizational readiness to change assessment subscale scores and implementation of hepatitis prevention services in substance use disorders treatment clinics: a case study. Implement. Sci. 2010;5:46. doi: 10.1186/1748-5908-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Owen R.R., Drummond K.L., Viverito K.M. Monitoring and managing metabolic effects of antipsychotics: a cluster randomized trial of an intervention combining evidence-based quality improvement and external facilitation. Implement. Sci. 2013;8:120. doi: 10.1186/1748-5908-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawk K.F., D’Onofrio G., Chawarski M.C. Barriers and facilitators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beebe J. Rapid assessment process. Encyclop. Social Measure. 2005:285–291. [Google Scholar]
- 58.Gale R.C., Wu J., Erhardt T. Comparison of rapid vs in-depth qualitative analytic methods from a process evaluation of academic detailing in the veterans health administration. Implement. Sci. 2019;14(1):11. doi: 10.1186/s13012-019-0853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hsieh H.F., Shannon S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 60.Curry L.A., Nembhard I.M., Bradley E.H. Qualitative and mixed methods provide unique contributions to outcomes research. Circulation. 2009;119(10):1442–1452. doi: 10.1161/CIRCULATIONAHA.107.742775. [DOI] [PubMed] [Google Scholar]
- 61.Tate J.P., Sterne J.A.C., Justice A.C. Veterans aging cohort S, the antiretroviral therapy cohort C. albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS. 2019;33(5):903–912. doi: 10.1097/QAD.0000000000002140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agency for Healthcare Research and Quality . 2019. Healthcare Cost and Utilization Project. [Google Scholar]
- 63.Veterans Health Administration . Jay and Cliff Smith and Office of Mental Health and Suicide Prevention; 2020. VA/DoD CLINICAL PRACTICE GUIDELINE FOR THE MANAGEMENT OF SUBSTANCE USE DISORDERS. [Google Scholar]
- 64.U.S. Department of Health and Human Services Drugs. https://aidsinfo.nih.gov/drugs (Accessed 3.17.2020) [DOI] [PubMed]
- 65.Justice A.C., McGinnis K.A., Tate J.P. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend. 2016;161:95–103. doi: 10.1016/j.drugalcdep.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams E.C., McGinnis K.A., Bobb J.F. Changes in alcohol use associated with changes in HIV disease severity over time: a national longitudinal study in the veterans aging cohort. Drug Alcohol Depend. 2018;189:21–29. doi: 10.1016/j.drugalcdep.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ritchie K.M., Miller C.J., Oliver K.A., Smith J.L., Lindsay J.A., Kirchner J.E. 18 May, 2017. Using Implementation Facilitation to Improve Care in the Veterans Health Administration (Version 2). [Google Scholar]
- 68.Ritchie M.J., Kirchner J.E., Townsend J.C., Pitcock J.A., Dollar K.M., Liu C.F. Time and organizational cost for facilitating implementation of primary care mental health integration. J. Gen. Intern. Med. 2020;35(4):1001–1010. doi: 10.1007/s11606-019-05537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woertman W., de Hoop E., Moerbeek M., Zuidema S.U., Gerritsen D.L., Teerenstra S. Stepped wedge designs could reduce the required sample size in cluster randomized trials. J. Clin. Epidemiol. 2013;66(7):752–758. doi: 10.1016/j.jclinepi.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Mugavero M.J., Westfall A.O., Zinski A. Measuring retention in HIV care: the elusive gold standard. J. Acquir. Immune Defic. Syndr. 2012;61(5):574–580. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.MacKinnon D.P., Lockwood C.M., Hoffman J.M., West S.G., Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGovern M.P., Carroll K.M. Evidence-based practices for substance use disorders. Psychiatr. Clin. N. Am. 2003;26(4):991–1010. doi: 10.1016/s0193-953x(03)00073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.National Resource Center for Academic Detailing (NaRCAD) 2020. https://www.narcad.org/ [Google Scholar]
- 74.Oldfield B.J., Muñoz N., Boshnack N. No more falling through the cracks: a qualitative study to inform measurement of integration of care of HIV and opioid use disorder. J. Subst. Abus. Treat. 2019;97:28–40. doi: 10.1016/j.jsat.2018.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WHAT-IF? Provider and Staff Survey (baseline)
WHAT-IF? Focus group guides
WHAT-IF? Implementation Facilitation Manual