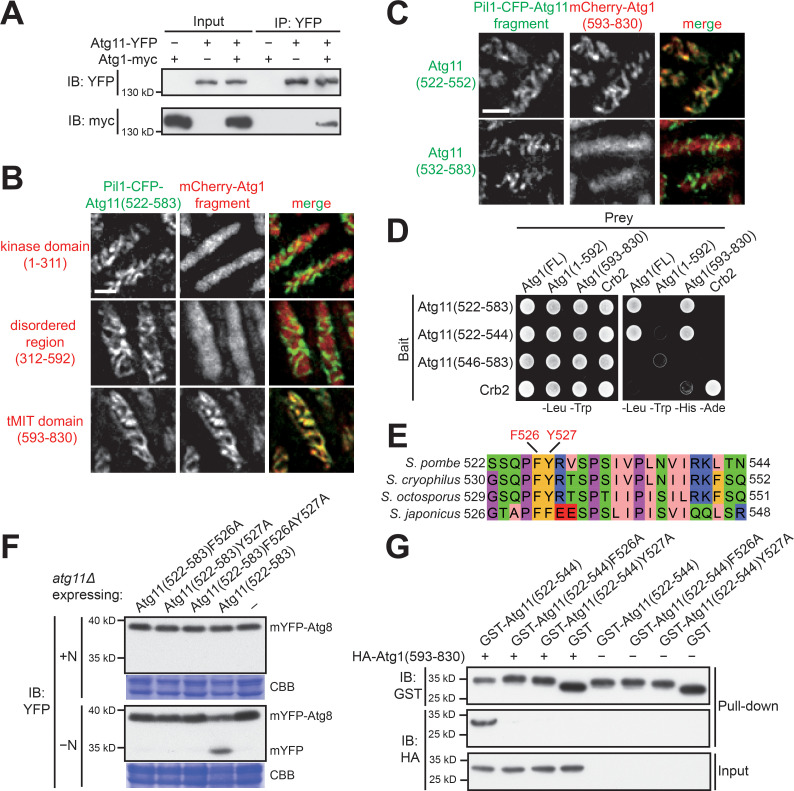
Figure 2. Atg11(522-544) mediates a specific and direct interaction with the tMIT domain of Atg1.
(A) Coimmunoprecipitation assay showed that Atg11 physically interacts with Atg1. Atg11 and Atg1 were endogenously tagged with YFP and myc, respectively. (B) Pil1 co-tethering assay identified an interaction between Atg11(522-583) and the tMIT domain of Atg1. Log-phase cells co-expressing Pil1-CFP-Atg11(522-583) and an mCherry-tagged Atg1 fragment were examined by fluorescence microscopy. Scale bar, 3 μm. (C) Atg11(522-552) but not Atg11(532-583) interacted with the tMIT domain of Atg1 in the Pil1 co-tethering assay. Scale bar, 3 μm. (D) Y2H assay showed that Atg11(522-544) is sufficient for interacting with Atg1. Crb2, which can self-interact, served both as a positive control and a specificity control. (E) The sequence of S. pombe Atg11(522-544) was aligned to the corresponding regions of Atg11 proteins from three other fission yeast species. (F) Mutating either F526 or Y527 to alanine disrupted the autophagy function of Atg11(522-583). mYFP-Atg8 processing assay was performed as in Figure 1A. (G) In vitro GST pull-down assay using recombinant proteins demonstrated a direct interaction between Atg11(522-544) and the tMIT domain of Atg1.