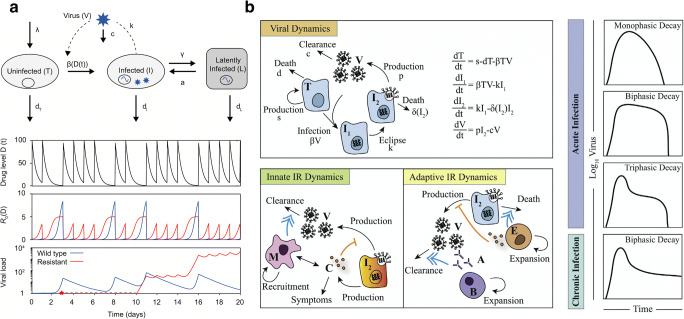
Fig. 4.
Viral modeling in HIV and influenza. (a) Hill et al. [73] described how an augmented viral dynamics model can be used to simulate antiretroviral therapy and the evolution of drug resistance in HIV. Uninfected cells (U) become infected (I) from infection by virus (V). Infected cells can become latently infected (L) and can be reactivated to produce actively infected cells. Latently infected cells are a crucial compartment to consider for long-term therapy outcomes as temporary administration of fully suppressive therapy can falsely be predicted to cure infection. Simulating an example of a drug taken daily with 70% probability, the impact on the viral load, and R0 for wild-type and resistant strains is significantly different over 20 days. Reprinted open access [73]. (b) Smith [91] summarized the major components of modeling viral infections, including innate and adaptive immune response (IR) dynamics. Viral dynamics are represented by an ODE viral kinetic model with an eclipse phase (I1) and an infected cell clearance which is a function of the infection cell population. Macrophages (M) play a major role in the innacte IR clearing up virus and also producing cytokines which block the production of new virions. In the adaptive immune response, T cells (E) play a major role in the clearance of infected cells with the addition of activated B cells (B) and antibody (A) production leading to eventual viral clearance. Viral load dynamics observed in actue and chronic infections can be significantly different depending on the underlying viral and IR kinetics. Reprinted from Current Opinion in Systems Biology, Volume 12, A.M. Smith, Validated Models of Immune Response to Virus Infection, Page 47, Copyright (2018), with permission from Elsevier