Abstract
Matrix Metalloproteinases (MMPs)-induced altered proteolysis of extracellular matrix proteins and basement membrane holds the key for tumor progression and metastasis. Matrix metalloproteinases-7 (Matrilysin), the smallest member of the MMP family also performs quite alike; thus serves as a potential candidate for anti-tumor immunotherapy. Conversely, being an endogenous tumor-associated antigen (TAA), targeting MMP-7 for immunization is challenging. But MMP-7-based xenovaccine can surmount the obstacle of poor immunogenicity and immunological tolerance, often encountered in TAA-based conventional vaccine for anti-tumor immunotherapy. This paves the way for investigating the potential of MMP-7-derived major histocompatibility complex (MHC)-binding peptides to elicit precise epitope-specific T-cell responses towards their possible inclusion in anti-tumor vaccine formulations. Perhaps it also ushers the path of achieving multiple epitope-based broad and universal cellular immunity. In current experiment, an immunoinformatics approach has been employed to identify the putative canine matrix matelloproteinases-7 (cMMP-7)-derived peptides with MHC class-I-binding motifs which can elicit potent antigen-specific immune responses in BALB/c mice. Immunization with the cMMP-7 DNA vaccine induced a strong CD8+ cytotoxic T lymphocytes (CTLs) and Th1- type response, with high level of gamma interferon (IFN-γ) production in BALB/c mice. The two identified putative MHC-I-binding nonameric peptides (Peptide32-40 and Peptide175-183) from cMMP-7 induced significant lymphocyte proliferation along with the production of IFN-γ from CD8+ T-cells in mice immunized with cMMP-7 DNA vaccine. The current observation has depicted the immunogenic potential of the two cMMP-7-derived nonapeptides for their possible exploitation in xenovaccine-mediated anti-tumor immunotherapy in mouse model.
Keywords: Immunoinformatics, Mammary tumor, Matrix metalloproteinase-7, Major histocompatibilty complex, Xenogeinic DNA vaccine
Introduction
Cancer stands next to cardiovascular disease as the second-leading cause of global death with an estimated accountability around 9.6 million deaths in 2018 [1]. Among several cancer types, breast cancer is the most common among women, affecting 2.1 million women each year with approximately 627,000 deaths in 2018 which is around 15% of overall cancer deaths accountability among women [1]. The incidence and mortality rate of breast cancer cases world widely is reported to be 11.6% and 6.6%, respectively. Further, it has been estimated that the total breast cancer cases per year will be 2,778,850 in 2040 as per the prediction of World Health Organisation (WHO) [2]. Surgery, radiotherapy and systemic therapy are the major therapeutic modalities to be employed alone or in combination considering the available resources and patient factors. For instance, efficacy analyses of chemotherapeutic agents such as doxorubicin and tamoxifen in presence of cholesterol-depleting agent methyl-β-cyclodextrin, and mitomycin C along with proteasomal inhibitor MG132 has depicted promising outcomes in pre-clinical models of several solid tumors [3–5]). Regulatory microRNAs (miRNAs)-based therapeutics which regulates key cancer signalling mediators has also yielded promising outcome in breast cancer suppression [6–8]. Several regulatory cytokines of diverse cancer signalling pathways can also serve as novel therapeutic checkpoints in targeted oncotherapy [9, 10]. Even the herbal products such as bitter melon extract, Ricinus communis L. fruit extract, Scrophularia atropatana extracts, etc. which produced promising results in restricting breast cancer growth in pre-clinical model, has been suggested as potential therapeutic module for cancer treatment [11–14]. However, cancer recurrence or spread along with general cytotoxicity often arises as post-therapeutic complications leading into poor prognosis. Thus, an ideal cancer treatment should have specificity to distinguish neoplastic and healthy cells and the potency to eradicate systemic tumors from the body. In this perspective, antigen-specific anti-angiogenesis and cancer immunotherapy represent two attractive approaches for efficient cancer treatment. The activation of antigen-specific CTL-mediated immune responses induce killing of tumor cells expressing specific tumor associated antigen (TAA) [15–18]; while inhibition of angiogenesis controls neoplastic growth by sequestering the malignant cells from adequate blood supply [19]. Hence, blending of both the approach may serve to elicit the most potent and precise antitumor effect.
Identification and targeting of several key type-specific TAAs have opened the avenue for precise immunotherapy of different tumor types in recent times. Matrix metalloproteinaseses-7 (MMP-7), cognominated as matrilysin is a crucial TAA having plethora of functions for tumor progression and metastasis. MMP-7 induced proteolysis of extracellular matrix (ECM) proteins and basement membrane paves the path for tumorigenic invasion [20–23]. Simultaneous modulation of several cancer signalling pathways related to inflammation, angiogenesis, cell proliferation, apoptosis and migration also produce synergistic effect towards tumor establishment and progression; thus rendering MMP-7 as key target for TAA-based tumor immunotherapy [24–26]. Despite the potential, the most common problem encountered with TAA-based cancer vaccine is most of such antigens are self and predominantly non-mutated proteins of patient’s own origin, thus poorly immunogenic as well as oftentimes obstacled by immunological tolerance to elicit significant immune responses [27–29]. Although TAAs are mostly evolutionarily conserved but the subtle interspecies structural variability can be exploited to develop xenovaccines for targeted immunotherapy which can surmount the inherent limitations of TAA-based vaccines. Significant success has been achieved in recent times by introducing such xenogenic homologous TAA proteins to induce potent immune response against their self endogenous counterpart [20–32]. Further xenogenic DNA vaccines can elicit T cell as well as antibody responses which adds extra edge over conventional autologic and allogenic cell-based vaccines and peptide tumor antigens [33–35]. Although the potency has yet to be a tested in human but xenovaccinotherapy has already achieved laboratory to clinical transition to serve as effective mean for the treatment of melanoma, renal cancer, tumors of digestive system, lung cancer and prostate cancer [36]. The scope of the current strategy can be extended towards customization of polyvalent or chimeric xenogenic cancer vaccines based upon fusion of strongly immunogenic epitopes from a single or multiple TAAs. MMP-7 peptides have been depicted to elicit CTL responses which recognize primary autologous leukemic cells and lyse them in a MHC-restricted and antigen-specific manner but spared normal B and T cells [37]. In current elucidation, two nonameric peptides (Peptide32-40 and Peptide175-183) from cMMP-7 with MHC class-I binding motifs were identified. The customized synthetic peptides were evaluated for their ability to induce a CTL response in vitro in BALB/c mice primed with the whole protein (cMMP-7 DNA Vaccine).
Materials and methods
Ethics statement
The animal experiments performed in the current study, were strictly adhered with the policy on the guidelines and approval of the Institute Animal Ethics Committee (IAEC) and Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), approval letter No. F. 26-1/2015–16/J.D (R). Mice were euthanized employing CO2, and every measure was extended to minimise the animal suffering.
Cell lines and culture condition
The HEK-293 T cell line was obtained from The National Centre for Cell Science (NCCS), Pune, India and adopted to grow in DMEM with L-Glutamine (2 mM), NaHCO3 (1.5 mM) and HEPES (10 mM) buffer (Himedia, India). All cultures were supplemented with 10% fetal bovine serum (Hyclone, USA) and 50 μg/ml of gentamycin (Gibco; Invitrogen, UK), and were kept in a humidified environment at 37 °C in the presence of 5% CO2, 95% air atmosphere. Cells were trypsinized using standard protocol for harvesting.
Plasmid, construction of DNA vaccines
MMP-7 gene from canine mammary tumor and murine MMP-7 was cloned in pVIVO2-mcs vector (InvivoGen, USA) for customizing xenogenic MMP-7 DNA vaccine constructs. The constructs were found to be functionally active as reported in our previous study [38, 39]. The recombinant pVIVO2.cMMP-7, pVIVO2.mMMP-7 and pVIVO2 vector were purified using EndoFree Plasmid Mega Kit (Qiagen, Germany) as per manufacture’s protocol, and purified products were re-suspended in normal saline followed by quantification using NanoDrop™ 1000 Spectrophotometer (Thermo Scientific, USA).
Experimental mice and immunization
The female Balb/c mice of about 4–5 weeks were purchased from National Centre for Laboratory Animal Sciences, Hyderabad, India. The animals were maintained at the Division of Biochemistry, Indian Veterinary Research Institute India. Mice were kept in well-fumigated house and polypropylene cages following strict hygienic measures under standard feeding and watering management [40]. In this study, three experimental groups were included viz. I (pVIVO2.mcs), II (pVIVO2.mMMP-7) and III (pVIVO2.cMMP-7) for immunization with respective DNA vaccine. Each group contained six mice which were inoculated intramuscularly with 100 μg of respective plasmid DNA and boosted with the same amount of DNA construct on days 14 and 28. Sera and splenocytes were collected 1 week after the last immunization. Vaccination with empty pVIVO2 vector served as the control.
Antigens and synthetic peptides
The full-length cMMP-7 was cloned into pET32a expression vector melded to a histidine (His) tag using primer pair 5′-CAGGATCCCAGGTCAGGACTATCTC-3′ and 5′-GCCGAAGCTTAAATTCTGT TCCCTCCGTATA-3′. The His-tagged recombinant protein was expressed in E. coli BL21 (DE3) and purified by Ni–NTA chromatography, as described previously [41]. The in-silico analyses of cMMP-7 protein sequence for identification of putative high-affinity binding nonapeptides with class I HLA and class I mouse MHC was performed using BIMAS [42] and SYFPEITHI [43] algorithms. The predictive analyses yielded two most eligible cMMP-7 derived peptides containing MHC class I-restricting motifs, 32FFRLPVTGI40 (Peptide32-40) and 175FLIAATHEL183 (Peptide175-183) which were custom synthesized (Hysel, India). The customized peptides were dissolved in distilled water to achieve a concentration of 10 μg/ml and stored at -70 °C till further use.
Quantification of IgG1 and IgG2a isotypes by ELISA
Serum IgG1 and IgG2a concentrations from the vaccinated animals after seven days of the last immunization were estimated in triplicate using an ELISA kit (eBioscience, USA) according to the manufacturer’s guidelines.
Lymphocyte transformation test (LTT) employing 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
Antigenic-specific multiplication of mice splenocytes was analysed by lymphocyte proliferation assay [44]. Briefly, at the seventh day after the last immunization, spleens from the mice were dissected out and disintegrated with a glass slide. The splenocytes were strained using a cell strainer (40 µM, BD Falcon, USA) and treated with 0.75% ammonium chloride buffer (pH 7.65) for 5 min to lyse red blood cells. The splenocytes (2 × 106/ml), were resuspended into RPMI1640 medium supplemented with 2 mM L-glutamine, 10% heat inactivated FCS, 50 U/ml penicillin, and 50 U/ml streptomycin. The cell suspensions (100 µl) were plated in triplicate, each for stimulated and un-stimulated control in 96-well culture plate and incubated with 10 µg/ml 32FFRLPVTGI40 (Peptide32-40) and 175FLIAATHEL183 (Peptide175-183) under IL-2 (20 IU/ml) stimulation for 72 h at 37 °C with 5% CO2. The MTT solution (20 µl at 5 mg/ml concentration) was added to each well and the plate was incubated for another 4 h at 37 °C. Subsequently the plate was centrifuged at 400 g for 10 min to discard the supernatant followed by addition of 150 µl DMSO and thorough mixing by pipetting. The solubilisation of formazan crystals was visualized under microscope and finally absorbance was recorded at 595 nm with iMark™ Microplate Absorbance Reader (Bio-Rad, USA).
Intracellular cytokine staining with flow cytometry analysis to detect CD8 + IFN-γ + T-cells
Seven days after the last immunization mice splenocytes were suspended (2 × 106/ml) in supplemented RPMI1640 medium containing 2 mM L-glutamine, 10% inactivated FBS, 50 U/ml penicillin, and 50 U/ml streptomycin. The cell suspensions (100 µl) were plated in 96-well culture plate (in triplicate) and incubated with 10 µg/ml 32FFRLPVTGI40 (Peptide32-40)/ 175FLIAATHEL183 (Peptide175-183) under IL-2 (20 IU/ml) stimulation for 72 h at 37 °C with 5% CO2. Then Brefeldin A (10 µg/ml; Sigma, USA) was added and incubated further for another 6 h. The cells were washed with RPMI1640 by centrifugation at 350 × g for 5 min and stained for surface CD8 molecules and intracellular INF-γ using conjugated mAbs [45]. Cells were stained for CD8 with FITC-conjugated rat anti-mouse CD8a mAbs (clone 53–6.7; eBioscience, USA) by adding 0.4 µg of each antibody for 1 × 106 cells, followed by incubation at room temperature for 30 min. Surface-stained cells were washed twice with washing solution (0.2% BSA and 0.2% sodium azide in PBS) and fixed with 4% paraformaldehyde at 4 °C for 15 min. Furthermore, the cells were permeabilized with 0.1% saponin (Sigma, USA) at 37 °C for 15 min followed by washing with 0.1% saponin buffer before staining for intracellular INF-γ with PE-Cy5-conjugated rat anti-mouse INF-γ mAb (Southern Biotech, USA) at 37 °C for 30 min. At last, the cells were washed thrice with washing buffer, fixed with 1% paraformaldehyde and stored at 4 °C until analysis by flow cytometry. The predicted frequency of CD8 + INF-γ + T-cells was determined by subtracting the percentage of unsensitized CD8 + INF-γ + T- cells from the percentage of antigen-sensitized CD8 + INF-γ + T- cells.
Statistical analysis
The data were subjected to analyses of variance (ANOVA) using the SPSS statistical package (SPSS for Windows 20 software). Differences among the data sets were tested with one-way ANOVA Duncan’s multiple range tests, and p < 0.05 was considered as level of significance.
Results
Cloning of cMMP-7 gene, expression and purification of recombinant cMMP-7 protein
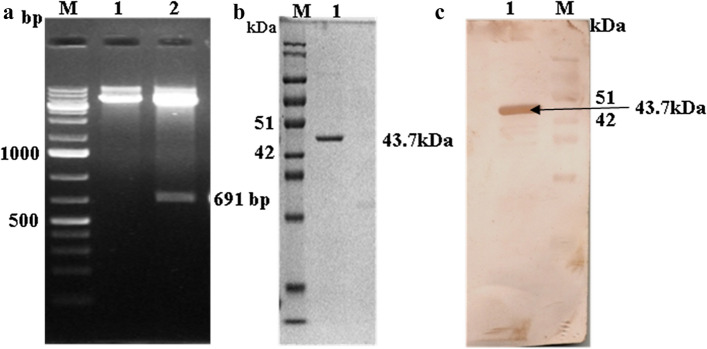
The amplified cMMP-7 gene fragment was cloned into pET32a expression vector and transformed into E. coli DH5α cells. Plasmids were isolated from the selected colonies and checked for the insert releasing by RE double digestion with EcoRI and HindIII. The recombinant plasmids released a 691 bp insert of desired size (Fig. 1a). The cMMP-7 mature peptide was recombinantly expressed in E. coli BL21(DE3) as a fusion protein with 6XHis tag having about 43.7 kDa (Fig. 1b) size. The specific reactivity of the Ni–NTA affinity purified recombinant protein was verified by western blotting (Fig. 1c) using rabbit anti-cMMP-7 hyperimmune sera available in the laboratory. The H2Kd prediction analysis indicated that canine MMP-7 32FFRLPVTGI40 (Peptide32–40) and 175FLIAATHEL183 (Peptide175–183) possessed significant binding affinity as high-scoring MHC class I (H2Kd) epitopes as compared to the other remaining peptides.
Fig. 1.
Cloning of canine MMP-7 (cMMP-7), Purification of recombinant cMMP-7 and Western blotting a Double endonuclease digestion of the recombinant vector pET-32a.cMMP-7: M-DNA maker; Lane 1: Undigested recombinent plasmide and lane 2- release of insert, digestion with EcoR1 and Hindlll b SDS-PAGE of purified r-cMMP-7 protein c Western blot of rMMP-7 by using Polyclonal antiserum raised against r-cMMP-7: M- protein ladder, lane 1- immunoblot at 43.7 kDa
Antibodies responses in immunized mice
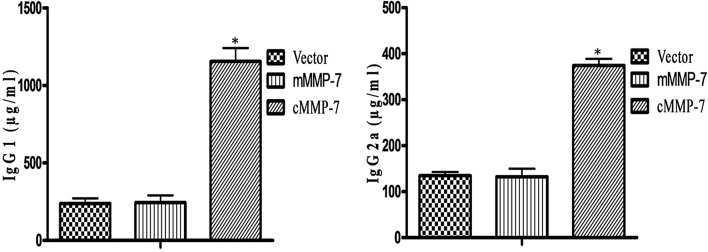
Mice from the cMMP-7 vaccinated group generated significantly higher levels of IgG antibody titer in comparison to the other vaccinated groups [39]. IgG subtype analyses indicated towards the type of immune response elicited by the DNA vaccine. The IgG1 and IgG2a subtype antibody levels in all the groups after seven days of the final immunization were depicted in the Fig. 2. Mice immunized with pVIVIO2.cMMP-7 DNA vaccine generated significantly (p < 0.05) higher levels of IgG1 (1166 ± 68.00 µg/ml) and IgG2a (373.1 ± 19.20 µg/ml) response in respect to the other two vaccinated groups: pVIVO2.mMMP-7 (IgG1: 233.85 ± 30.15 µg/ml and IgG2a: 129.55 ± 2.55 µg/ml) or pVIVO2mcs vector control (IgG1: 281.9 ± 16.50 µg/ml and IgG2a: 120.05 ± 1.95 µg/ml). All the data were presented as Mean ± SEM.
Fig. 2.
Antibody isotyping secretion in BALB/c mice immunized with cMMP-7: Bar diagram showing effect of treatment on immunoglobulin and presented as Mean ± SEM diagram. Significant difference was observed between treated and vector control. However effect of treatment was not differing from the control. *p < 0.05, level of significance
Lymphocyte proliferation assay
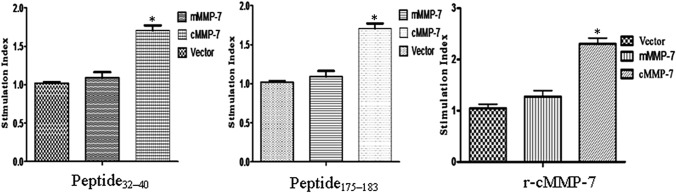
Lymphocyte proliferation assay demonstrated the T-lymphocyte proliferation indicating the cellular immune responses. The pVIVO2.cMMP-7 vaccinated group of mice showed significantly (p < 0.01) higher stimulation index in presence of recombinant-cMMP-7 as well as both the cMMP-7 derived peptides, 32FFRLPVTGI40 (Peptide32–40) and 175FLIAATHEL183 (Peptide175–183); whereas no significant difference was observed between the pVIVO2.mMMP-7 and pVIVO2 empty vector control groups (Fig. 3).
Fig. 3.
In vitro T cell proliferative response specific for the cMMP-7 protein and peptides: cMMP-7 DNA primed splenocytes were restimulated in vitro with r-cMMP-7 protein (20 µg/ml) and chosen synthetic peptides (Peptide32–40 and Peptide175–183) respectively with concentration of (10 µg/ml) and tested for T-cell proliferation. Results are presented as Mean ± SEM. *p < 0.01, level of significance
Antigen-specific proliferation and INF-γ production by cMMP-7-specific T-cell lines
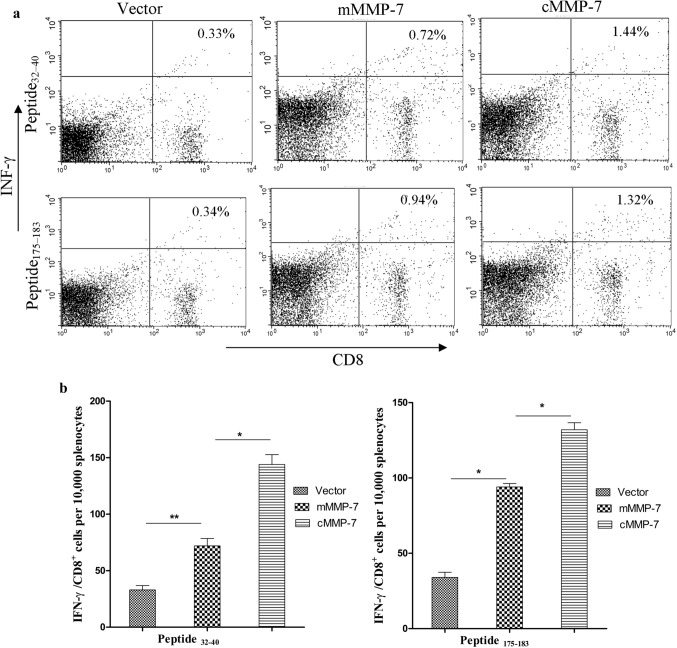
Seven days after the last vaccination, splenocytes from each group were incubated with cMMP-7 derived peptides, 32FFRLPVTGI40 (Peptide32–40) and 175FLIAATHEL183 (Peptide175–183). The percentage of antigen-specific IFN-γ producing CD8 + T -cells following immunization with different DNA vaccines were depicted in Fig. 4a, b. Immunization with canine MMP-7 based xenogeneic DNA vaccine significantly (P < 0.05) increased antigen-specific IFN-γ producing CD8 + T -cells in primed splenocytes as compared to both pVIVO2.mMMP-7 and pVIVO2 empty vector control groups.
Fig. 4.
Expression of IFN-γ from stimulated CD8 + BALB/c splenocytes: a Intracellular IFN-γ staining of antigen-specific T cells following in vitro stimulation with synthetic peptides. Primed T cells were expended in vitro in the presence of the synthetic peptide (10 µg/ml) and percentage of CD8 + T cells secreting IFN-γ was determined by intracellular staining of IFN-γ, b Bar diagram showing IFN-γ /CD8 + cells per 10,000 splenocytes presented as Mean ± SEM, mice vaccinated with cMMP-7 showed a marked increase in IFN-γ /CD8 + cells compared to the control groups (*p < 0.05)
Discussion
Xenovaccine-mediated immunotherapy is ushering the path for a paradigm shift in oncotherapy from the conventional approaches towards a target-specific safe option alleviating the issues of resistance and general cytotoxicity inherent to chemotherapy. The strategy employing cMMP-7 as TAA have already seemed to untie the knots of poor immunogenicity, tumor induced immunosupression and immunological tolerance to elicit strong NK activity, CTL responses along with up-regulating Th1 cytokine production in the murine breast cancer model which reduced the tumor growth and increased the survival of the diseased mice. The experiment employed whole recombinant cMMP-7 protein for inducing the immune system of the mice vaccinated with pVIVO2.cMMP7 plasmid alone or co-administration with pVIVO2.IL-18 plasmid [39]. Similar approach has yielded success as evidenced by xenogeneic vaccine plasmid DNA encoding human tyrosinase initiated an immune response against the TAA tyrosinase [46]. Several reports depicting the efficacy of xenogeneic vaccines using different TAAs in mouse models also second the concept, such as telomerase reverse transcriptase [47], human N′-terminal neu DNA vaccine [48], human tumor endothelial marker 8 DNA vaccine [49], and prostatic acid phosphatase dendritic cell-based vaccine [50]. However, further introspection was required to justify that instead of the entire recombinant xenoantigen, whether certain immunodominant peptide can serve the purpose. Fusion of multiple such immunodominant epitopes from a single or multiple TAA scan create the avenue for the development of polyvalent or chimeric xenogenic cancer vaccines. In current study, an immunoinformatics approach have been employed to identify cMMP-7 derived peptides with high-affinity MHC class-I binding motifs which can elicit potent antigen-specific T-cell responses in BALB/c mice because an effective vaccine against malignant tumor should enhance cellular immune response, mediated by induction of IFN-γ secreting cells and CD8+ CTLs [51]. Several studies have revealed that MHC class I-restricted CD8 + T cells recognise cancerous cells and release IFN-γ, which kills the cancerous cells, although engagement of both innate as well as adaptive arms of immune system may be indispensable to achieve sustainable tumor regression in metastatic cases [52, 53]. In the present work, we used two of these identified putative T-cell epitopes (Peptide32–40 and Peptide175–183) from the cMMP-7 protein and demonstrated that they were recognized by CD8+ T cells from cMMP-7 DNA-immunized mice. Previously, we observed that vaccination utilizing the xenogeneic cMMP-7 naked DNA vaccine via intramuscular route generated a robust antibody response against mMMP-7 protein. These anti-cMMP-7 antibodies were found to be both highly abundant and specific in comparison to vector and mMMP-7 controls. Further, the vaccine also induced cellular immune responses as evidenced by significant lymphocyte proliferation, immunoglobulin secretions and T-cytotoxic lymphocyte responses in BALB/c mice; thus, proving the potential of cMMP-7 as an effective candidate against mammary tumor [39].
The pVIVIO2.cMMP-7 DNA vaccine induced profound antibody response in the vaccinated animals. The IgG subtype analyses depicted significantly (p < 0.05) higher levels of IgG1 and IgG2a levels in cMMP-7 vaccinated animals as compared to the other two groups indicating towards a cMMP-7 DNA vaccine-induced mixed type of Th1/Th2 immune response. In-vitro restimulation of the isolated splenocytes from the mice immunized with pVIVO2.cMMP-7 DNA vaccine with either purified whole recombinant cMMP-7 protein or the customized cMMP-7 derived synthetic peptides depicted that the peptides as well as the intact recombinant protein induced substantial lymphocyte proliferation as significantly (p < 0.01) higher stimulation index was observed in presence of recombinant cMMP-7 protein and both of the derived peptides in cMMP-7 vaccinated group only. This indicated antigen-specific clonal expansion of a sub-set of immune cells towards initiation of the specific immune responses in the vaccinated animals. Further, antigen-specific proliferation of IFN-γ+T-cells and CD8+-IFN-γ+ T-cells in primed splenocytes along with enhanced IFN- γ production in presence of both the peptides only in cMMP-7 vaccinated group clearly depicted that the cMMP-7 derived putative MHC I binding peptides were successful to mimic the in-vivo natural processing of c-MMP-7 and its subsequent presentation to elicit a Th1-polarized response. The preferential induction of Th1-type cytokines is interesting as this may lead to the activation of macrophages and natural killer cells to execute the cancerous cells selectively, and may also promote the activation and proliferation of antigen specific CD8+ CTLs [54, 55]. The potential of the cMMP-7 derived peptides may be exploited to generate epitope-based xenovaccines for anti-tumor immunotherapy. Perhaps it may facilitate the exploitation of advantages associated with synthetic peptide-based vaccines over entire antigen-based vaccines such as easy packaging in smaller delivery vehicles, possibility of achieving multiple epitope-based broad and universal cellular immunity, etc. in future.
Although, the cMMP-7 epitope-based xenovaccine has yielded promising outcome, however elucidation of the potential immunotoxic effects urges further introspection. The adverse-effects of anti-MMP-7 immunotherapy is yet to be elucidated vividly but human MMP-9 and MMP-2 derived synthetic peptide-based vaccine has significantly restricted tumor growth in mice melanoma without producing any pathological side-effects [56]. Further, peptide‐based vaccine immunotherapy targeting several TAAs has also depicted promising outcomes against urological and colorectal cancer without producing any severe adverse effects [57–59]. In contrast, T-cell immunotherapy using engineered T cell receptor targeted against carcinoembryonic antigen produced significant regression of metastatic colorectal cancer but also induced severe colitis [60]. Thus, a keen attention is required to ensure the safety profile of such vaccines. The adverse effects of the vaccine in pregnancy also requires further investigation as inhibition of MMPs may affect uterine growth and expansion leading to premature labor [61]. However, oral delivery of anti-angiogenic listeriolysin-mVEGFR2 DNA vaccine targeting the VEGF receptor which carries out important functions in peri-implantation angiogenesis during early pregnancy has produced no adversity in establishment and progression of healthy pregnancy [62, 63].
Conclusion
In conclusion, the present introspection has vividly depicted that the putative cMMP-7 derived MHC class I binding nonapeptides have the potential to mimic its corresponding whole antigen counterpart in terms of in-vivo customary antigen processing and presentation to elicit a Th1-polarized response. Considering the xenoantigenic potential of cMMP-7, the acquired knowledge can be replicated to customize single or multiple epitope-based chimeric xenovaccines towards targeted tumor immunotherapy.
Acknowledgements
The authors are thankful to the Director, ICAR-Indian Veterinary Research Institute Izatnagar-243122, UP, India for providing necessary facilities and providing fund (Grant No. IVRI/BIOCHEM/11-14/003) to carry out the work.
Compliance with ethical standards
Conflict of interest
Authors declare they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.who.int/news-room/fact-sheets/detail/cancer (12/09/ 2018)
- 2.https://www.paho.org/hq/index.php/global-cancer-profile (2020)
- 3.Mohammad N, Malvi P, Meena AS, et al. Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol Cancer. 2014;13:204. doi: 10.1186/1476-4598-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad N, Singh SV, Malvi P, et al. Strategy to enhance efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci Rep. 2015;5:11853. doi: 10.1038/srep11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SV, Ajay AK, Mohammad N, et al. Proteasomal inhibition sensitizes cervical cancer cells to mitomycin C-induced bystander effect: the role of tumor microenvironment. Cell Death Dis. 2015;6:e1934. doi: 10.1038/cddis.2015.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muhammad N, Bhattacharya S, Steele R, Ray RB. Anti-miR-203 suppresses ER-positive breast cancer growth and stemness by targeting SOCS3. Oncotarget. 2016;7:58595–58605. doi: 10.18632/oncotarget.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivas MA, Venturutti L, Huang YW, Schillaci R, Huang TH, Elizalde PV. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res. 2012;14:R77. doi: 10.1186/bcr3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang CF, Shi ZM, Li DM, et al. Estrogen-induced miR-196a elevation promotes tumor growth and metastasis via targeting SPRED1 in breast cancer. Mol Cancer. 2018;17:83. doi: 10.1186/s12943-018-0830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Chouhan S, Mohammad N, Bhat MK. Resistin causes G1 arrest in colon cancer cells through upregulation of SOCS3. FEBS Lett. 2017;591:1371–1382. doi: 10.1002/1873-3468.12655. [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi K, Sakurai M, Yamamoto Y, et al. Alteration of specific cytokine expression patterns in patients with breast cancer. Sci Rep. 2019;9:2924. doi: 10.1038/s41598-019-39476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhammad N, Steele R, Isbell TS, Philips N, Ray RB. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget. 2017;8:66226–66236. doi: 10.18632/oncotarget.19887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shim SH, Sur S, Steele R, et al. Disrupting cholesterol esterification by bitter melon suppresses triple-negative breast cancer cell growth. Mol Carcinog. 2018;57:1599–1607. doi: 10.1002/mc.22882. [DOI] [PubMed] [Google Scholar]
- 13.Majumder M, Debnath S, Gajbhiye RL, et al. Ricinus communis L. fruit extract inhibits migration/invasion, induces apoptosis in breast cancer cells and arrests tumor progression in vivo. Sci Rep. 2019;9:14493. doi: 10.1038/s41598-019-50769-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safarzadeh E, Delazar A, Kazemi T, et al. The cytotoxic and apoptotic effects of scrophularia atropatana extracts on human breast cancer cells. Adv Pharm Bull. 2017;7:381–389. doi: 10.15171/apb.2017.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MA, Luo Y, O’Donnell M, et al. Development and preclinical evaluation of a Bacillus Calmette-Guérin-MUC1-based novel breast cancer vaccine. Cancer Res. 2003;63:1280–1287. [PubMed] [Google Scholar]
- 16.Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, de la Rosa C, Cheever MA. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein–based vaccine. J Clin Oncol. 2004;22:1916–1925. doi: 10.1200/JCO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Lladser A, Ljungberg K, Tufvesson H, Tazzari M, Roos AK, Quest AF, Kiessling R. Intradermal DNA electroporation induces survivin-specific CTLs, suppresses angiogenesis and confers protection against mouse melanoma. Cancer Immunol Immunother. 2010;59:81–92. doi: 10.1007/s00262-009-0725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stauss HJ, Thomas S, Cesco-Gaspere M, Hart DP, Xue SA, Holler A, King J, Wright G, Perro M, Pospori C, Morris E. WT1-specific T cell receptor gene therapy: improving TCR function in transduced T cells. Blood Cells Mol Dis. 2008;40:113–116. doi: 10.1016/j.bcmd.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Luo ST, Shi HS, Li M, Zhang HL, He SS, Liu Y, Pan Y, Yang L. AAV2-mediated gene transfer of VEGF-Trap with potent suppression of primary breast tumor growth and spontaneous pulmonary metastases by long-term expression. Oncol Rep. 2012;28:1332–1338. doi: 10.3892/or.2012.1915. [DOI] [PubMed] [Google Scholar]
- 20.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–290. doi: 10.1007/s00726-010-0689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int J Mol Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiomi T, Lemaître V, D’Armiento J, Okada Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol Int. 2010;60:477–496. doi: 10.1111/j.1440-1827.2010.02547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med. 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa T, Kimura Y, Hirano H, Higashi S. Matrix metalloproteinase-7 induces homotypic tumor cell aggregation via proteolytic cleavage of the membrane-bound Kunitz-type inhibitor HAI-1. J Biol Chem. 2017;292:20769–20784. doi: 10.1074/jbc.M117.796789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan S, Lin L, Gan R, et al. Elevated matrix metalloproteinase 7 expression promotes the proliferation, motility and metastasis of tongue squamous cell carcinoma. BMC Cancer. 2020;20:33. doi: 10.1186/s12885-020-6521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itoh K, Yamada A, Mine T, Noguchi M. Recent advances in cancer vaccines: an overview. Jpn J Clin Oncol. 2009;39:73–80. doi: 10.1093/jjco/hyn132. [DOI] [PubMed] [Google Scholar]
- 28.Slingluff CL, Jr, Speiser DE. Progress and controversies in developing cancer vaccines. J Transl Med. 2005;3:18–26. doi: 10.1186/1479-5876-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochenderfer JN, Gress RE. A comparison and critical analysis of preclinical anticancer vaccination strategies. Exp Biol Med. 2007;232:1130–1141. doi: 10.3181/0702-MR-42. [DOI] [PubMed] [Google Scholar]
- 30.Bergman PJ, Camps-Palau MA, McKnight JA, et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine. 2006;24:4582–4585. doi: 10.1016/j.vaccine.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 31.Walters JN, Ferraro B, Duperret EK, et al. A novel DNA vaccine platform enhances neo-antigen-like T cell responses against WT1 to break tolerance and induce anti-tumor immunity. Mol Ther. 2017;25:976–988. doi: 10.1016/j.ymthe.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potebnya GP, Symchych TV, Lisovenko GS. Xenogenic cancer vaccines. Exp Oncol. 2010;32:61–65. [PubMed] [Google Scholar]
- 33.Srinivasan R, Wolchok JD. Tumor antigens for cancer immunotherapy: therapeutic potential of xenogeneic DNA vaccines. J Transl Med. 2004;2:12. doi: 10.1186/1479-5876-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seledtsov VI, Shishkov AA, Surovtseva MA, et al. Xenovaccinotherapy for melanoma. Eur J Dermatol. 2006;16:655–661. [PubMed] [Google Scholar]
- 35.Seledtsov VI, Niza NA, Felde MA, Shishkov AA, Samarin DM, Seledtsova GV, Seledtsov DV. Xenovaccinotherapy for colorectal cancer. Biomed Pharmacother. 2007;61:125–130. doi: 10.1016/j.biopha.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Seledtsovaa GV, Shishkova AA, Kaschenkoa EA, Seledtsovb VI. Xenogeneic cell-based vaccine therapy for colorectal cancer: safety, association of clinical effects with vaccine-induced immune responses. Biomed Pharmacother. 2016;83:1247–1252. doi: 10.1016/j.biopha.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama Y, Grünebach F, Schmidt SM, Heine A, Häntschel M, Stevanovic S, Rammensee HG, Brossart P. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14:5503–5511. doi: 10.1158/1078-0432.CCR-07-4041. [DOI] [PubMed] [Google Scholar]
- 38.Yadav PK, Gupta SK, Kumar S, Saini M, Mishra SR, Nandakumar P, Katari M. Characterization and in vitro expression studies of a potential xenogeneic DNA vaccine against canine mammary tumours. Indian J Anim Sci. 2017;87:1480–1484. [Google Scholar]
- 39.Yadav PK, Gupta SK, Kumar S, Ghosh M, Yadav BS, Kumar D, Kumar A, Saini M, Katari M. IL-18 immunoadjuvanted xenogeneic canine MMP-7 DNA vaccine overcomes immune tolerance and supresses the growth of murine mammary tumor. Int Immunopharmacol. 2020;82:106370. doi: 10.1016/j.intimp.2020.106370. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council (US) Committee for the update of the guide for the care and use of laboratory animals . Guide for the care and use of laboratory animals. 8. Washington, D.C.: National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 41.Yadav PK, Sunil Kumar BV, Chanu VKh, Yadav BS, Kumar A, Kataria M. Recombinant tissue inhibitor of metelloproteinase-3 from canine mammary tumor induces apoptosis in-vitro. Indian J Anim Sci. 2015;85:588–592. [Google Scholar]
- 42.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 43.Rammensee H, Bachmann J, Emmerich NP, Bachor OA, Stevanovic S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 44.Sawant PM, Verma PC, Subudhi PK, Chaturvedi U, Singh M, Kumar R, Tiwari AK. Immunomodulation of bivalent Newcastle disease DNA vaccine induced immune response by co-delivery of chicken IFN-γ and IL-4 genes. Vet Immunol Immunopathol. 2011;144:36–44. doi: 10.1016/j.vetimm.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 45.Chaitra MG, Shaila MS, Nayak R. Characterization of T-cell immunogenicity of two PE/PPE proteins of Mycobacterium tuberculosis. J Med Microbiol. 2008;57:1079–1086. doi: 10.1099/jmm.0.47565-0. [DOI] [PubMed] [Google Scholar]
- 46.Grosenbaugh DA, Leard AT, Bergman PJ, et al. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am J Vet Res. 2011;72:1631–1638. doi: 10.2460/ajvr.72.12.1631. [DOI] [PubMed] [Google Scholar]
- 47.Adotévi O, Mollier K, Neuveut C, et al. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T-cell immunity in vivo. Blood. 2010;115:3025–3032. doi: 10.1182/blood-2009-11-253641. [DOI] [PubMed] [Google Scholar]
- 48.Tu CF, Lin CC, Chen MC, Ko TM, Lin CM, Wang YC, Lai MD. Autologous neu DNA vaccine can be as effective as xenogenic neu DNA vaccine by altering administration route. Vaccine. 2007;25:719–728. doi: 10.1016/j.vaccine.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Ruan Z, Yang Z, Wang Y, Wang H, Chen Y, Shang X, Yang C, Guo S, Han J, Liang H, Wu Y. DNA vaccine against tumor endothelial marker 8 inhibits tumor angiogenesis and growth. J Immunother. 2009;32:486–491. doi: 10.1097/CJI.0b013e3181a1d134. [DOI] [PubMed] [Google Scholar]
- 50.Olson BM, Frye TP, Johnson LE, Fong L, Knutson KL, Disis ML, McNeel DG. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatise. Cancer Immunol Immunother. 2010;59:943–953. doi: 10.1007/s00262-010-0820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Sullivan T, Saddawi-Konefka R, Vermi W, et al. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J Exp Med. 2012;209:1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haabeth OA, Lorvik KB, Hammarström C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat Commun. 2011;2:240. doi: 10.1038/ncomms1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moynihan KD, Opel CF, Szeto GL, et al. Eradication of large established tumors in mice by combination immunotherapy that engages innate and adaptive immune responses. Nat Med. 2016;22:1402–1410. doi: 10.1038/nm.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, Ossendorp F, Melief CJ, Arens R. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189:3397–3403. doi: 10.4049/jimmunol.1201540. [DOI] [PubMed] [Google Scholar]
- 56.Roomi MW, Niedzwiecki A, Rath M (2018) Peptide vaccines directed against human metalloproteinases (MMPs) with anti-tumor efficacy in vitro and in vivo. J Cell Med Nat Health (CM&NH) 1
- 57.Obara W, Kanehira M, Katagiri T, Kato R, Kato Y, Takata R. Present status and future perspective of peptide-based vaccine therapy for urological cancer. Cancer Sci. 2018;109:550–559. doi: 10.1111/cas.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi N, Ohkuri T, Homma S, Ohtake J, Wakita D, Togashi Y, Nishimura T. First clinical trial of cancer vaccine therapy with artificially synthesized helper/killer-hybrid epitope long peptide of MAGE-A4 cancer antigen. Cancer Sci. 2012;103:150–153. doi: 10.1111/j.1349-7006.2011.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kavanagh B, Ko A, Venook A, et al. Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother. 2007;30:762–772. doi: 10.1097/CJI.0b013e318133451c. [DOI] [PubMed] [Google Scholar]
- 60.Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011;19(3):620–626. doi: 10.1038/mt.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Khalil RA. Matrix Metalloproteinases in normal pregnancy and preeclampsia. Prog Mol Biol Transl Sci. 2017;148:87–165. doi: 10.1016/bs.pmbts.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matejuk A, Leng Q, Chou ST, Mixson AJ. Vaccines targeting the neovasculature of tumors. Vascular Cell. 2011;3:7. doi: 10.1186/2045-824x-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Douglas NC, Tang H, Gomez R, Pytowski B, Hicklin DJ, Sauer CM, Zimmermann RC. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology. 2009;150:3845–3854. doi: 10.1210/en.2008-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]