Table 1.
Current in vitro microfluidic platforms enable researchers to precisely control the microenvironment through incorporation of various cell types, ECM components, and mechanical stimuli, while maintaining compatibility with a wide range of established readouts.
| In vitro Model Type | Advantages/Disadvantages | Specific examples | Applications for investigating cross-talk in infections |
|---|---|---|---|
| Transwell/cell culture inserts | + Simple and robust + Compatible with standard cell culture equipment + Standard air-liquid interface protocol + No external equipment requirement (e.g., pumps or vacuum for controlled fluid or air flow) + Compatible with established readouts (e.g., microscopy, RNA analysis) + Access to cultures for direct manipulation − Limited customization − Mechanically static − Poorly quantified diffusion gradients |
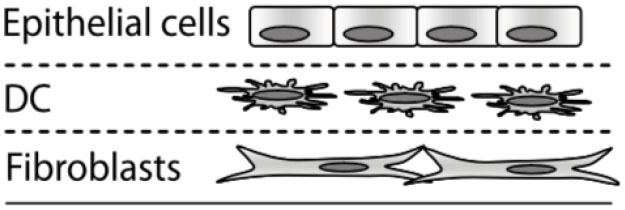
(189)
|
• Direct contact signaling • Soluble factor signaling • Co-culture with various cell types (e.g., DCs, macrophages, stromal cells) • Air-liquid interface on 3D culture • Cell-permeable inserts can be used for neutrophil/immune cell migration modeling through epithelium |
| Mechanically “breathing” microfluidic model | + Cyclic airflow and mechanical stretching possible + Continuous nutrient/media flow + Customizable + ALI compatible + Compatible with established readouts (e.g., microscopy, RNA analysis) + Control over diffusion gradients − External equipment required (e.g., syringe pump for fluid flow control and vacuum pump for pressure control) − Microfabrication technology may be necessary for additional customization − Difficult to access cultures for direct manipulation |
(188, 221)“Lung-on-a-Chip”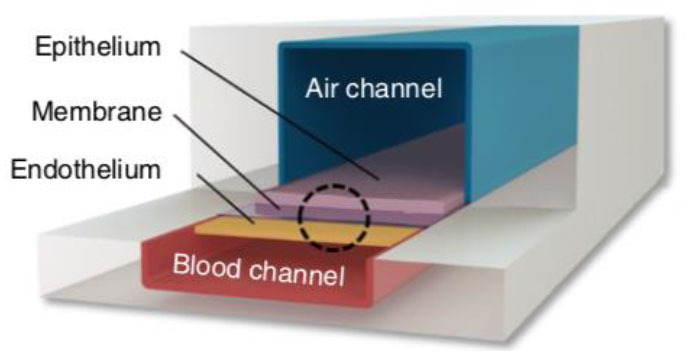
|
• Direct contact signaling • Soluble factor signaling • Investigations involving mechanical stretch • Investigations requiring controlled air or liquid flow • Coculture with multiple cell types (e.g., endothelium) • Porous membrane allows for neutrophil/immune cell migration modeling through epithelium • Control over airflow in ALI model |
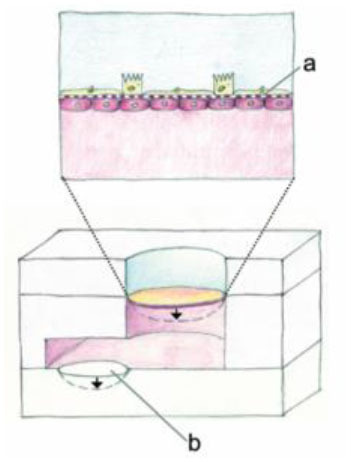
(223)
|
• Direct contact signaling • Soluble factor signaling • Investigations involving mechanical stretch • Investigations requiring controlled air or liquid flow • Coculture with multiple cell types (e.g., endothelium) • Porous membrane allows for neutrophil/immune cell migration modeling through epithelium • Open well for easier access of culture for downstream analysis |
||
| Hydrogel-based microfabricated models | + Customizable + Complexity in tissue components and shape + Multiple signaling modes (i.e., volatile, direct contact, soluble factor, etc.) + ALI compatible + Open systems enable access to cultures for direct manipulation − External equipment may be required if controlled fluid flow or airflow is desired − Microfabrication technology may be necessary for additional customization |
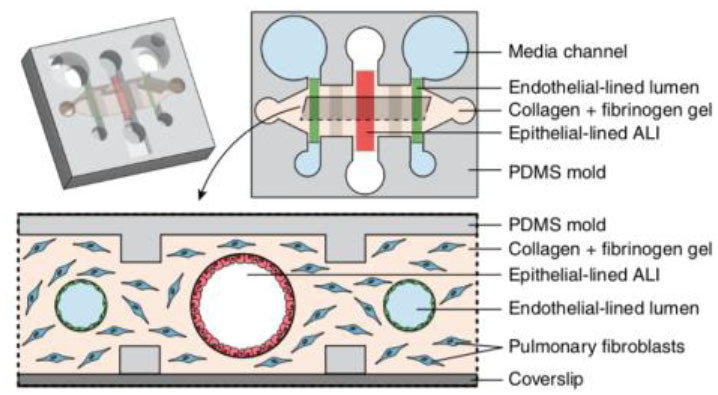
(178)
|
• Volatile signaling coculture experiments • Direct contact signaling • Soluble factor signaling • Investigations involving endothelial, mesenchyme, and epithelial cells • Modular cell culture experiments • Co-infection experiments |
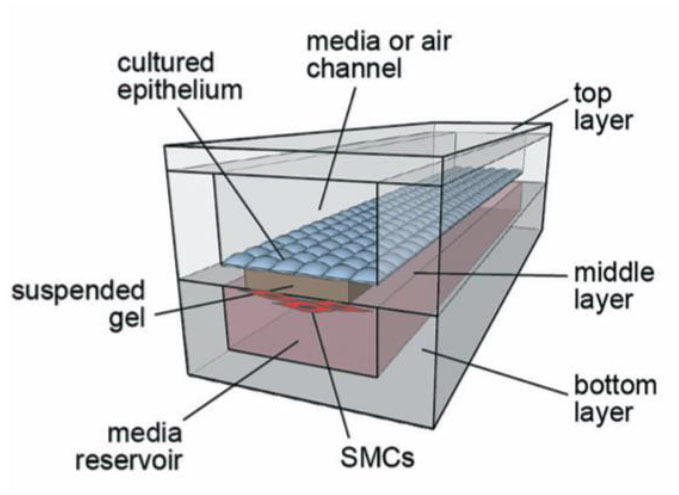
(226)
|
• Co-culture with epithelial and smooth muscle cells • Native ECM interactions (i.e., collagen/matrigel) • Continuous media flow • Air liquid interface • Soluble factor signaling • Direct contact signaling |
Various model types can be adapted for different biological systems, dependent on which components of the lung microenvironment need to be examined.
