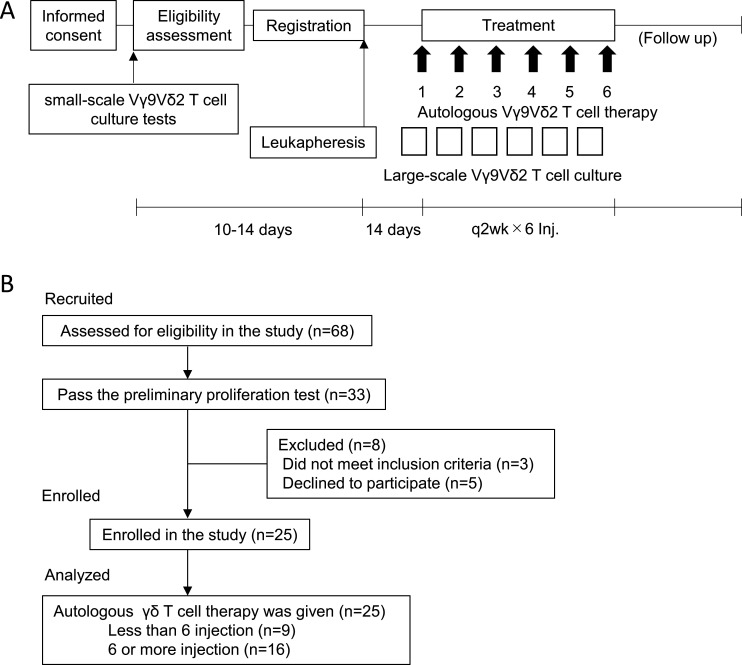
Figure 1.
Study design and participant flow chart. (A) Study design of autologous Vγ9Vδ2 T-cell transfer therapy. After informed consent, small-scale Vγ9Vδ2 T-cell culture testing was performed to examine the feasibility of Vγ9Vδ2 T-cell expansion for therapeutic use. Eligible patients were leukapheresed and expanded Vγ9Vδ2 T-cells were infused back to the same patient every 2 weeks. (B) Participant flow chart. Twenty-five patients were enrolled in the study.