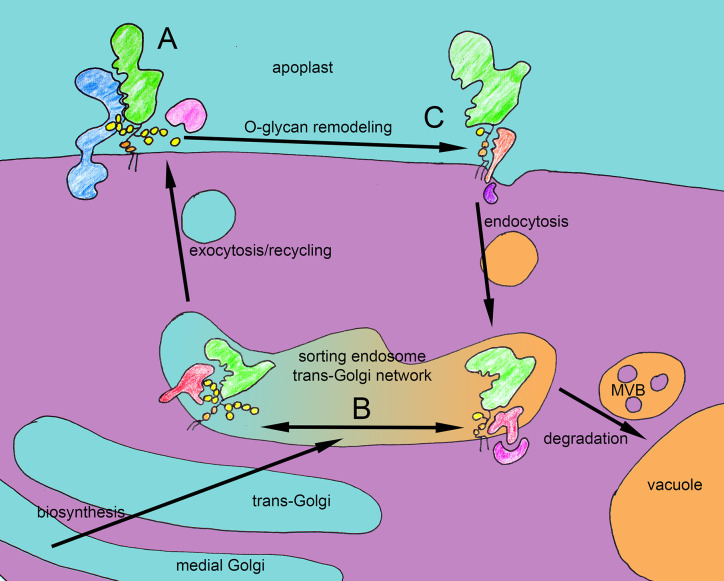
Figure 2.
Hypothetical functions of O-glycans of FLA4 in trafficking and receptor binding. Three potential scenarios that might explain recent genetic and cell biological observations. (A) The O-glycan (yellow) might be required for interaction between FLA4 (green) and FEI receptor kinases (blue). (B) In the secretory pathway the O-glycan might be checked by a system of different cargo receptors (red) that either bind fully or incompletely O-glycosylated FLA4 and their cytosolic adaptors (purple). While the fully glycosylated FLA4 molecules are rapidly transported to the cell surface via exocytosis, the hypoglycosylated species are targeted for destruction to the vacuoles via multivesicular bodies. (C) Full O-glycosylation might stabilize FLA4 at the cell surface and as binding partner for receptor kinases while its apoplastic remodeling by glycanases (pink) might make the protein competent to be recruited by endocytotic cargo receptors. The number and identity of the involved molecular components are unknown and despite their colorful depiction, the symbols for receptors and cytosolic adaptors represent “black boxes” at the moment.