Highlights
-
•
Cross-Sectional study on Posttraumatic Stress Disorder (PTSD) with two control groups.
-
•
Higher fractional anisotrophy (FA) in forceps minor (FM) in trauma controls.
-
•
Higher volume in the left and right anterior insulae in trauma controls.
-
•
Positive correlations between the FA in the FM and the volume of the insulae.
-
•
Negative correlations between the FA in the FM and symptom severity of PTSD.
Keywords: White and gray matter, PTSD, Trauma, Diffusion tensor imaging, Neuroplasticity
Abstract
Differences in structural white and gray matter in survivors of traumatic experiences have been related to the development and maintenance of Posttraumatic Stress Disorder (PTSD). However, there are very few studies on diffusion tensor imaging and region based morphometry comparing patients with PTSD to two control groups, namely healthy individuals with or without trauma experience. It is also unknown if differences in white and gray matter are associated. In this cross-sectional study, we examined white- and gray matter differences between 44 patients with PTSD, 49 trauma control and 61 healthy control subjects. We compared the groups applying Tract-Based Spatial Statistics (TBSS) for a whole brain white matter analysis as well as region of interest analyses for white and gray matter. First, trauma control subjects in comparison to patients with PTSD and healthy control subjects showed significantly a) higher fractional anisotropy (FA) in the left corticospinal tract and inferior fronto-occipital fasciculus than patients with PTSD, b) higher FA in the left inferior fronto-occipital-, right inferior– and right superior longitudinal fasciculi, c) higher FA in the forceps minor and d) higher volume of the left and right anterior insulae. Second, we show significant correlations between the FA in the forceps minor and the gray matter volume in the left and right anterior insulae. Third, the mean FA value in the forceps minor correlated negatively with symptom severity of PTSD and depression as well as trait anxiety, whereas the gray matter volume in the left anterior insula correlated negatively with symptom severity in PTSD. Our findings underline the importance of brain structures critically involved in emotion regulation and salience mapping. While previous studies associated these processes primarily to functional and task-based differences in brain activity, we argue that morphometrical white and gray matter differences could serve as targets in neuroscientifically-informed prevention and treatment interventions for PTSD.
1. Introduction
The experience of a traumatic event can lead to the development of Posttraumatic Stress Disorder (PTSD). The fifth edition of the Diagnostic and Statistical Manual (DSM-5; American Psychiatric Association, 2013) characterizes PTSD by four symptom clusters: a) the re-experience of the traumatic event in form of intrusions or flashbacks; b) avoidance behavior around thoughts, feelings or reminders of the event; c) negative alterations in cognitions and mood; d) heightened arousal and reactivity. In the past two decades, a large amount of neuroimaging studies investigated differences in brain morphology in patients with PTSD when compared to either healthy individuals with or without trauma experience (Bromis et al., 2018, Daniels et al., 2013, Kühn and Gallinat, 2013, Siehl et al., 2018). The volumetric change of regions in the brain is an important indicator for underlying disease mechanisms and potential target regions for interventions. However, only few studies include more than one comparison group, with studies either focusing on a sample of healthy control subjects (HC) or a sample of healthy individuals that have experienced at least one traumatic event, so called trauma control subjects (TC). Choosing one or the other as a comparison group leads to very different results and conclusions. We therefore include both control groups in our study comparing patients with PTSD to TC and HC subjects. Furthermore, white and gray matter differences are largely studied independently and possible associations between them are rarely discussed within a common theoretical framework. We would like to bridge the gap between imaging techniques by studying structural differences in white- and gray matter within a single sample. By studying multiple imaging modalities, we aim to draw conclusions on how these differences are interrelated and how novel prevention and intervention tools might benefit from multiple outcome variables.
An estimated half of the brain volume consists of white matter with short and long reaching fibers passing on information (Sampaio-Baptista and Johansen-Berg, 2017). An important mechanism of the human brain is the ability of white matter tracts to change during maturation of the human brain (Giedd and Rapoport, 2010, Lövdén et al., 2010) or when learning occurs (Scholz et al., 2009, Wang and Young, 2014, Zatorre et al., 2012), a process called white matter plasticity (Sampaio-Baptista and Johansen-Berg, 2017). White matter plasticity also plays an important role in the development of anxiety disorders in general (Jenkins et al., 2016) and PTSD in particular (Daniels et al., 2013, Siehl et al., 2018). Recent meta-analyses comparing patients with PTSD and TCs and HCs showed mixed results with lower and higher fractional anisotropies (FA) in patients in the anterior and posterior part of the cingulum, the superior longitudinal fasciculus and frontal white matter tracts, such as the forceps minor and the uncinate fasciculus (Daniels et al., 2013, Siehl et al., 2018). As argued in more detail in Siehl et al. (2018), alterations in the above mentioned white matter tracts in patients with PTSD might be associated with context learning, processing of emotionally salient cues and extinction of aversive memories. However, as mentioned before, most studies focused on the comparison between patients with PTSD and trauma control subjects, with only a single study comparing patients with PTSD to trauma and healthy control subjects (Sun et al., 2013). Information on structural white matter differences between patients with PTSD and HCs as well as TC and HC subjects are still scarce, and a more refined understanding is needed to further establish neural white matter tracts as markers following trauma exposure. This also includes a link to several clinical target measures, such as PTSD characteristics and comorbidity, which can co-determine these effects (Ginzburg et al., 2010).
Similar to white matter plasticity, there is gray matter plasticity due to axon sprouting, dendritic branching, neuro- or angiogenesis or changes in glia cells following for example experience based learning (Kühn et al., 2014, Zatorre et al., 2012). We can quantify volumetric gray matter differences by a technique called voxel-based morphometry (Ashburner and Friston, 2005), in which the volume of voxels in the whole brain is estimated and can be compared between groups. This approach can also be applied to particular regions of interest (ROIs) estimating regional volumes with a so called region-based morphometry (RBM; Gaser and Dahnke, 2016). Recent meta-analyses found a reduction in overall brain volume in patients with PTSD in comparison to trauma and healthy control subjects with the largest differences in the volume of the insulae, the hippocampi and the anterior cingulate cortices and the superior frontal gyri in ROI analyses and the medial prefrontal- and the anterior cingulate cortices in the whole brain voxel-based morphometry analysis (VBM; Kühn and Gallinat, 2013, Bromis et al., 2018). In a large meta-analysis of 89 studies, Bromis et al. (2018) showed accumulated evidence of 38 studies reporting differences in the volume of the hippocampi, showing differences in the following three contrasts: patients with PTSD < HC subjects, patients with PTSD < TC subjects, TC < HC subjects. Furthermore, they reported a reduction of gray matter volume of the insulae between patients with PTSD < HC as well as between patients with PTSD < TC. This fits well to psychobiological models suggesting a downregulation of brain activity in areas associated to processing context information, such as the hippocampal formation, and emotion regulation in more frontal parts of the brain and an upregulation of brain activity in areas associated to salience processing and threat detection, in areas such as the insulae and amygdalae (Brewin et al., 2010, Liberzon and Abelson, 2016).
In the present study, we analyzed data from a large civilian sample including patients with PTSD, TCs, and HCs. We expected higher FA in frontal white matter, such as the forceps minor and uncinate fasciculus in TC and HC subjects in comparison to patients with PTSD. Further, we expected higher gray matter volumes of the hippocampi in HC subjects in comparison to TCs and patients with PTSD, between TC subjects and patients with PTSD as well as HCs and TCs. We also expected higher gray matter volume of the anterior insulae in HC and TC subjects in comparison to patients with PTSD. We further explored gray matter differences in the following region of interests: amygdalae, posterior insulae, anterior and posterior cingulate cortices as well as the ventromedial prefrontal cortices (vmPFC). We expected a positive association between differences in white and gray matter. Finally, we expected significant negative correlations between symptom severity of PTSD and depression as well as trait anxiety and differences in white and gray matter volume.
2. Methods and materials
2.1. Participants
The dataset in this study is pooled from three independent studies on key mechanisms of pavlovian learning in patients suffering from PTSD (Wicking et al., 2016; two studies are in preparation). The studies were performed between 2010 and 2018 and all imaging protocols used for white and gray matter assessment were identical. In total, 154 participants were included in this study with 44 patients with PTSD, 49 TC and 61 HC subjects (see Table 1). There were no significant between-group differences observed for sex and age.
Table 1.
Demographic and clinical characteristics of study sample.
[Abbreviations: ADS – Allgemeine Depressionsskala [Centre for Epidemiological Studies Depression Scale (CESD)]; CAPS – Clinician-Administered PTSD Scale; HC – Group of healthy control subjects, who have never experienced anything traumatic in their lives; M – mean; SD – Standard deviation; STAI-T – State-Trait Anxiety Inventory – Trait Anxiety; TC – Group of trauma control subjects, who have at least experienced one traumatic event but do not fulfill the criteria for PTSD; 1Psychopharmacology: Aripiprazole, Pregabalin, Methylphenidate, Mirtazapine, Quetiapine, Sertraline, Trimipramine, Venlafaxine; 2Non-Psychopharmacological: Etoricoxib, Bisoprolol, Beta-Blocker, Ibuprofen, Metamizole, Levothyroxine].
| Variable |
Groups |
Analysis |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD N = 44 |
TC N = 49 |
HC N = 61 |
|||||||||||||||||
| M | SD | n | (%) | M | SD | n | (%) | M | SD | n | (%) | X2 | F | Df | p | ||||
| Sex (female) | 22 | 50.0 | 26 | 53.1 | 27 | 44.3 | 0.88 | 0.64 | |||||||||||
| Age (in years) | 42.8 | 11.2 | 41.1 | 13.6 | 43.0 | 13.9 | 0.02 | 1 | 0.88 | ||||||||||
| Education | <=12 years | 23 | 52.3 | 14 | 28.6 | 19 | 31.1 | 6.82 | 0.03 | ||||||||||
| >12 years | 21 | 47.7 | 35 | 71.4 | 42 | 68.9 | |||||||||||||
| Time since trauma (in years) | 13.0 | 9.2 | 15.0 | 9.1 | – | – | 0.95 | 1 | 0.33 | ||||||||||
| CAPS | 66.7 | 18.9 | 7.2 | 8.8 | – | – | 375.8 | 1 | <0.001 (PTSD > TC) | ||||||||||
| ADS | 28.4 | 9.7 | 9.8 | 7.6 | 7.0 | 4.8 | 142.2 | 1 | <0.001 (PTSD > TC) | ||||||||||
| STAI-T | 55.6 | 10.5 | 36.1 | 10.7 | 31.8 | 8.2 | 115.8 | 1 | <0.001 (PTSD > TC + HC) | ||||||||||
| Medication | Total (yes) | 29 | 65.9 | 19 | 38.8 | 24 | 39.3 | ||||||||||||
| Psychopharmacological1 | 21 | 7 | 4 | 9.08 | 0.01 | ||||||||||||||
| Non-Psychopharmacological2 | 8 | 12 | 20 | ||||||||||||||||
| Total (no) | 15 | 34.1 | 30 | 61.2 | 37 | 60.7 | |||||||||||||
| Type of traumatic event (index trauma) | Caused voluntarily | Total (caused voluntarily) | 27 | 61.4 | 21 | 42.9 | – | 3.18 | 0.08 | ||||||||||
| (1) Imprisonment | 2 | 1 | – | ||||||||||||||||
| (2) Physical violence | 8 | 4 | – | ||||||||||||||||
| (3) Sexual abuse | 1 | 1 | – | ||||||||||||||||
| (4) Rape | 3 | 1 | – | ||||||||||||||||
| (5) Wartime experience | 10 | 4 | – | ||||||||||||||||
| (6) Witness of sudden death/ serious injury of so. | 3 | 7 | – | ||||||||||||||||
| (8) Other experiences | 0 | 3 | – | ||||||||||||||||
| Caused involun-tarily | Total (caused involuntarily) | 17 | 38.6 | 28 | 57.1 | – | |||||||||||||
| (1) Natural disaster | 0 | 0 | – | ||||||||||||||||
| (2) Fire or explosion | 4 | 2 | – | ||||||||||||||||
| (3) Accident | 8 | 19 | – | ||||||||||||||||
| (4) Sudden death of so. | 2 | 2 | – | ||||||||||||||||
| (5) Other experiences | 3 | 5 | – | ||||||||||||||||
Participants in all groups, including subjects in the HC group, were asked if they had experienced any traumatic event from a list of possible traumatic events, taken from the Posttraumatic Diagnostic Scale (Foa, 1995, Foa et al., 1997). Then, the Structured Clinical Interviews for DSM-IV-TR (American Psychiatric Association, 2000) I and II were carried out for each participant (SCID; Fydrich et al., 1997, Wittchen et al., 1997) to assess PTSD, depression and other possible comorbidities. Participants fulfilling the PTSD criteria in the SCID-I interview were assigned to the PTSD group. To verify the assignment in a second step patients with PTSD had to fulfill criteria B through F of the DSM-IV criteria in the German version of the Clinician-Administered Posttraumatic Stress Disorder Scale (CAPS; Blake et al., 1995, Schnyder and Moergeli, 2002). This second step was independent of the overall score in the CAPS.
The following exclusion criteria applied: any traumatic experience before the age of 18 years, comorbid current or lifetime psychotic symptoms, current alcohol/ drug dependence or abuse, borderline personality disorder, cardiovascular or neurological disorders, brain injury, acute pain, continuous pain or medication for attention deficit hyperactivity disorder, pregnancy and metal implants.
For patients with PTSD and individuals in the TC group, no significant differences were present in time since trauma. The groups differed significantly in the level of education and medication with patients suffering from PTSD taking more psychopharmacological medication than participants in the control groups. All participants in the TC and HC groups that reported the intake of psychopharmacological medication were prescribed this medication for other purposes than a diagnosed mental disorder (e.g. sleep disturbances). Patients with PTSD scored significantly higher on symptom severity of PTSD and depression as well as trait anxiety than both control groups.
All participants received a reimbursement for participation (10€/h) and travel expenses. Patients were offered treatment in the outpatient clinics of the Central Institute of Mental Health in Mannheim, if requested. The study was carried out conforming to the Code of Ethics of the World Medical Association (World Medical Association, Declaration of Helsinki, seventh revision, 2013). The study was approved by the Ethical Review Board of the Medical Faculty Mannheim, Heidelberg University and all participants gave written informed consent including consent for data re-analysis.
2.2. Data acquisition
Whole-brain MRI images were acquired using a 3 T Magnetom TRIO whole body magnetic resonance scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a standard 12-channel volume head coil. We obtained T1-weighted, magnetization-prepared, rapid-acquisition gradient echo (MPRAGE) images with the following parameters: TR = 2300 ms, TE = 2.98 ms, flip angle 9°, FOV: 256 × 256 mm2, matrix size: 256 × 256, voxel size: 1.0 × 1.0 × 1.1 mm3, 160 sagittal slices. For the diffusion images we applied a single shot echo-planar imaging sequence (TR = 14000 ms, TE = 86 ms, 64 axial slices, 2 mm slice thickness, FOV: 256 × 256 mm2, matrix size: 128 × 128 mm2), with one image without diffusion weighting and 40 diffusion-weighted images (b = 1000 s/mm2) along forty non-collinear directions.
2.3. Preprocessing
2.3.1. White matter
First, the diffusion-weighted raw data were preprocessed using the Oxford Centre for Functional MRI of the Brain Software Library (FMRIB; FSL, version 6.0), UK; http://www.bmrib.ox.ac.uk/fsl; Behrens et al., 2003). The preprocessing procedure included the following steps: a) correction for motion artefacts and eddy current distortions using the FMRIB Diffusion Toolbox (FDT); b) extraction of the skull from T1-images using the Brain Extraction Tool (BET); c) fitting diffusion tensors at each voxel independently to the data and calculation of FA maps using a DTI fit algorithm, with alignment to the MNI space. In a second step, we extracted the mean FA value for each of the twenty white matter tracts specified by the probabilistic JHU white-matter tractography atlas (Mori et al., 2005) for a ROI analysis. The probability threshold was set to 30%, meaning that each voxel contained the corresponding tract with a 30% probability. We assessed motion parameters and included participants up to a maximum translation of one mm in x-, y-, or z-direction and a maximum of 1° of any angular motion throughout the course of the scan. No participants were excluded due to motion during the scan of white matter.
2.3.2. Gray matter
Second, we preprocessed the T1 weighted images using the Computational Anatomy Toolbox (CAT12; http://www.neuro.uni-jena.de/cat). The CAT12 toolbox runs on Statistical Parametric Mapping (SPM12; Wellcome Department of Imaging Neuroscience, London, UK) implemented in MATLAB R2016a (The MathWorks Inc., Natick, MA, USA). The preprocessing included the following steps: a) spatial registration; b) segmentation into gray and white matter and CSF; c) bias correction of intensity non-uniformities. The Neuromorphometric atlas (provided by Neuromorphometrics, Inc., MA, USA; http://www.neuromorphometrics.com) was chosen providing a total of 142 ROIs. In a second step, we extracted the mean gray matter volume for each subject for 14 predefined ROIs including both hippocampi, amygdalae, anterior and posterior insulae, anterior and posterior cingulate cortices as well as ventromedial prefrontal cortices. We assessed motion parameters and included participants up to a maximum translation of one mm in x-, y-, or z-direction and a maximum of 1° of any angular motion throughout the course of the scan. No participants were excluded due to motion during the scan of gray matter.
2.4. Analyses of structural brain data
2.4.1. White matter: Whole brain analysis
First, Tract-based spatial statistics (TBSS) was applied for the analysis of voxelwise FA changes (Smith et al., 2006) using FSL software. TBSS projects the FA data of all subjects onto a mean FA tract skeleton, before applying voxelwise cross-subject statistics. For statistical testing we conducted a one-way, univariate analysis of covariance (ANCOVA) with permutation-based nonparametric inference on FA with age and sex as nuisance covariates. FSL’s randomize was used with threshold-free cluster enhancement (TFCE; Smith and Nichols, 2009) and 5000 permutations per analysis to assess group differences between patients with PTSD, TC and HC subjects. For whole-brain multiple comparison correction, the statistical threshold was set at α < 0.05 with family-wise error (FWE) correction at cluster level (cluster threshold p < 0.001; Table 2).
Table 2.
Results of the whole brain cluster analysis of FA values (TBSS). ANCOVA includes comparison of all three experimental groups (patients with PTSD, TCs, HCs) and sex and age as covariates. Tracts were extracted according to the JHU white matter tractography atlas.
[Abbreviations: ATR – Anterior thalamic radiation; CST – Corticospinal tract; IFOF – Inferior fronto-occipital fasciculus; ILF – Inferior longitudinal fasciculus; L – Left; n.c. – not classified; R - Right; SLF – Superior longitudinal fasciculus].
| Contrast |
Cluster index |
voxels |
Significance |
Peak voxel coordinate |
Tracts |
||
|---|---|---|---|---|---|---|---|
| p | x | y | z | ||||
| ANCOVA | 2 | 46 | 0.046 | −27 | −25 | 17 | l CST |
| 1 | 24 | 0.045 | −25 | 28 | 12 | l IFOF | |
| Post-Hoc T-test (TC > HC) | 7 | 28,264 | 0.009 | −24 | 28 | 10 | l IFOF |
| 6 | 1,492 | 0.034 | 45 | −22 | −1 | r ILF | |
| 5 | 233 | 0.048 | 50 | −46 | 0 | r SLF | |
| 4 | 34 | 0.05 | −19 | −31 | 36 | l ATR | |
| 3 | 22 | 0.05 | 36 | −54 | −8 | r IFOF | |
| 2 | 17 | 0.05 | 35 | −49 | 8 | r IFOF | |
| 1 | 1 | 0.05 | 37 | −51 | −8 | r IFOF | |
| Post-Hoc T-test (TC > PTSD) | 11 | 23,058 | 0.014 | −27 | −26 | 17 | l CST |
| 10 | 305 | 0.046 | 31 | −33 | 14 | r IFOF | |
| 9 | 116 | 0.049 | 35 | −46 | 7 | r IFOF | |
| 8 | 109 | 0.049 | 39 | −44 | −11 | r ILF | |
| 7 | 85 | 0.049 | 47 | −25 | 4 | r ILF | |
| 6 | 30 | 0.049 | 32 | −44 | −15 | n.c. | |
| 5 | 22 | 0.05 | 40 | −39 | −11 | r ILF | |
| 4 | 19 | 0.05 | 57 | −18 | 3 | r ILF | |
| 3 | 15 | 0.05 | 37 | −38 | 15 | r SLF | |
| 2 | 2 | 0.05 | 40 | −53 | 1 | r ILF | |
| 1 | 1 | 0.05 | 27 | −40 | −18 | n.c. | |
2.4.2. White matter: ROI analysis
In a second step, we averaged the FA value across all voxels in each of the twenty white matter ROIs. The group difference of the mean FA of each tract between patients with PTSD, TC and HC subjects was assessed with 20 different ANCOVAs (one for each tract) with age and sex entered as nuisance variables. Bonferroni-corrections were applied across 20 tracts (significant at α < 0.0025). In case the ANCOVA showed a significant group difference, Post-hoc t-tests were performed using Tukey’s honestly significant difference (Tuckey’s HSD) test as post-hoc single-step multiple comparison procedure.
2.4.3. Gray matter
We performed a RBM analysis on the gray matter data. RBM estimates a regional tissue volume for different regions in the brain based on a surface-based atlas. We took fourteen pre-defined ROIs (each bilateral: hippocampi, amygdalae, anterior and posterior insulae, anterior and posterior cingulate cortices, ventromedial prefrontal cortices) from results of two meta-analyses (Bromis et al., 2018; Kühn and Gallinat, 2013). The group difference of the mean volume of each ROI between patients with PTSD, TC and HC subjects was assessed with fourteen different ANCOVAs (one for each tract) with age, sex and TIV entered as nuisance variables. Bonferroni-corrections were applied across 14 tracts (significant at α < 0.0036). Identical to the analyses steps in white matter, in case of a significant group difference, post-hoc t-tests were performed using Tukey’s honestly significant difference (Tuckey’s HSD) test as post-hoc single-step multiple comparison procedure.
2.4.4. Correlation of white and gray matter differences
In a final step, we carried out a Pearson’s product moment correlation to assess the association between the FA value in white matter tracts and the volume of ROIs in which groups differed significantly. We applied Bonferroni corrections dividing the p-value by the number of correlations that were performed.
2.5. Clinical assessments
Posttraumatic Stress Disorder. The German version of the CAPS (Blake et al., 1995, Schnyder and Moergeli, 2002) was used to provide a categorical diagnosis of PTSD and to assess symptom severity, which is calculated by summing the frequency and intensity score, measured on two 5-point scales ranging from zero (“never”/ “none”) to four (“most or all of the time”/ “extreme”). The CAPS score ranges from 0 to 100.
Depression. For the assessment of impairment due to depressive symptoms within the last week, we applied the German long version of the Center for Epidemiological Studies Depression Scale (ADS; Hautzinger and Bailer, 1993). The ADS is a self-report questionnaire with 20 items measured on a 4-point scale ranging from zero (“rarely or not at all (less than one day)”) to three (“most often, all of the time (on five to seven days)”). The ADS score ranges from 0 to 60.
Trait anxiety. For the assessment of trait anxiety, we applied the German version of the trait-version of the State-Trait-Anxiety-Inventory (STAIT; Laux et al., 1981). The STAI-T is a self-report questionnaire including 20 questions, measured on a 4-point Likert scale ranging from one (“not at all”) to four (“very much”). Higher scores are associated with higher levels of anxiety. The STAIT score ranges from 20 to 80.
2.6. Statistical analysis
All statistical analyses were performed in R-Statistics (R CoreTeam, 2013). Data were assessed for outliers and normal distribution. All assumptions were met, if not mentioned otherwise below. Analyses of covariance (ANCOVA) were computed including age and sex (for DTI and RBM) as well as total intracranial volume (TIV; for RBM). In case of multiple comparisons (e.g. multiple FA comparisons of different white matter tracts) Bonferroni corrections were applied to counteract Type 1 errors. We applied Tukey’s honestly significant difference (Tuckey’s HSD) test as post-hoc single-step multiple comparison procedure. Missing data were excluded from the analyses. However, this applied only to the gray matter analyses, in which seven datasets were missing (nPTSD = 2; nTC = 2, nHC = 3) due to incomplete measurements or artefacts. Correlations were calculated based on Pearson’s product moment correlation coefficient. This applied to correlations calculated between clinical assessments of PTSD, depression and trait anxiety and differences in white and gray matter volume as well as for correlations between differences in gray and white matter volume.
3. Results
3.1. White matter: Whole brain analysis
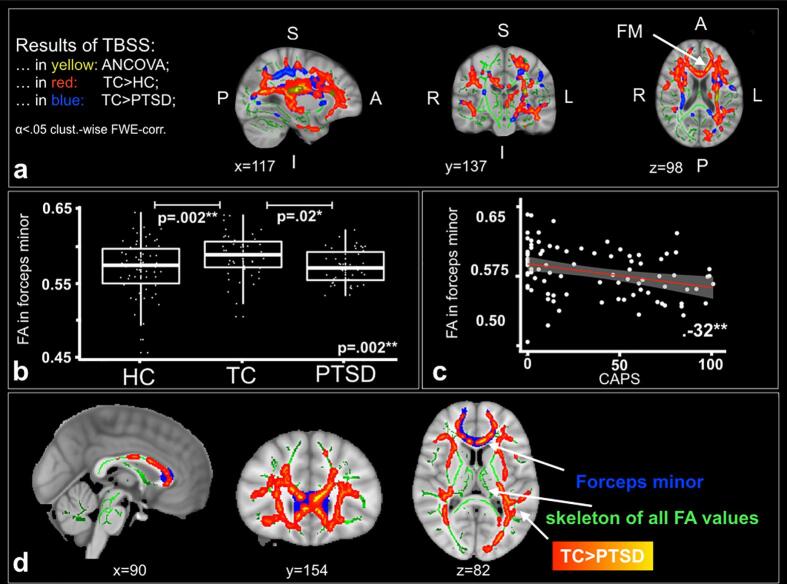
Fig. 1a illustrates significant between-group differences in FA (α ≤ 0.05 cluster-wise FWE-correction) based on a whole-brain TBSS, including age and sex as covariates. We found two significant clusters in the ANCOVA comparing all three groups. The first cluster was located in the left corticospinal tract (CST; cluster size k = 46 voxels, p = .046, MNI: x = -27, y = -25, z = 17) and the second cluster in the left inferior fronto-occipital fasciculus (IFOF; cluster size k = 24 voxels, p = .045, MNI: x = -25, y = 28, z = 112). The post-hoc contrast TC > HC resulted in seven clusters showing significantly different FA values with the three largest being the following (Fig. 1; Table 2): a) left IFOF (cluster size k = 28264 voxels, p = .009, MNI: x = -24, y = 28, z = 10), b) right inferior longitudinal fasciculus (ILF; cluster size k = 1492 voxels, p = .034, MNI: x = 45, y = –22, z = -1) and c) right superior longitudinal fasciculus (SLF; cluster size k = 233 voxels, p = .048, MNI: x = 50, y = -46, z = 0). A second post-hoc contrast TC > PTSD resulted in eleven clusters showing significantly different FA values with the three largest being the following (Fig. 1; Table 2): a) left CST (cluster size k = 23058 voxels, p = .014, MNI: x = -27, y = -26, z = 17), b) right IFOF (cluster size k = 305 voxels, p = .046, MNI: x = 31, y = –33, z = 14) and c) right IFOF (IFOF; cluster size k = 116 voxels, p = .049, MNI: x = 35, y = -46, z = 7). There was no significant difference between patients suffering from PTSD and HC subjects.
Fig. 1.
Diffusion Tensor Imaging. a) Results of TBSS analyses comparing the fractional anisotropy between patient with PTSD (n = 44), TC (n = 49) and HC (n = 61) subjects in an ANCOVA (yellow), between TC>HC subjects in a post-hoc t-test (red) and between TC>PTSD in a post-hoc t-test (blue). Age and sex were included as covariates in the analyses. All results are FWE-corrected (α <.05). b) Boxplots with significant (αbonferroni_cor =.05/20 =.0025) differences in mean FA value of the forceps minor between patients with PTSD (n = 44), TC (n = 49) and HC (n = 61) subjects (n = 154). c) Significant correlation (αbonferroni_cor =.05/4 =.0125) between mean FA value in the forceps minor and the mean CAPS score for TC subjects (n = 49) and patients with PTSD (n = 44). d) Anatomical images with mean FA skeleton used for the TBSS analysis (in green). The contrast between TC subjects and patients with PTSD is marked in yellow to red. The forceps minor is marked in blue as a region of interest for clarification.
[Abbreviations: ANCOVA - Analysis of Covariance; CAPS - Clinician-Administered PTSD Scale; FA - Fractional anisotropy; FM - Forceps minor; FWE - Family-wise error correction; HC - Healthy control subjects; I - Inferior; L - Left; P - Posterior; PTSD - Patients with posttraumatic stress disorder; R - Right; S - Superior; TBSS - Tract-based spatial statistics; TC - Trauma control subjects; * α <.05; ** α <.01; *** α <.001].
3.2. White matter: ROI analysis
When extracting 20 ROIs, one for each white matter tract, we found a significant difference in the mean FA of the forceps minor (F(2,149) = 6.56, p = .002; pbonf.cor. = 0.04). Post-hoc t-tests showed a significantly higher mean FA in the forceps minor for TC compared to HC (MDifference = 0.02; 95% CI 0.005 to 0.029; p = .002; Hedges’ g = 0.53) and TC compared to patients with PTSD (MDifference = 0.02; 95% CI 0.002 to 0.028, p = .02; Hedges’ g = 0.62). There was no significant difference between patients suffering from PTSD and HC (Fig. 1; Table 3). The white matter dataset comprised 154 participants.
Table 3.
Tract by tract comparisons of FA values between all three experimental groups (patients with PTSD, TCs, HCs). An ANCOVA was performed for each individual tract including age and sex as covariates.
*αbonferroni_cor=.05/20 =.0025;
[Abbreviations: ATR – Anterior thalamic radiation; CST – Corticospinal tract; FA – Fractional anisotropy; HC – Healthy control subjects; IFOF – Inferior fronto-occipital fasciculus; ILF – Inferior longitudinal fasciculus; PTSD – Subjects suffering from posttraumatic stress disorder; SLF – Superior longitudinal fasciculus; TC – Trauma control subjects]
| Anatomical region |
Hemisphere |
Groups |
Analyses |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD N = 44 |
TC N = 49 |
HC N = 61 |
Fgroup |
df |
p |
contrast |
Difference |
CI [−95%; +95%] |
PTukey HSD |
Hedges’c |
|||||
| M | SD | M | SD | M | SD | ||||||||||
| ATR | Left | 0.498 | 0.020 | 0.504 | 0.022 | 0.496 | 0.024 | 1.89 | 2 | 0.15 | |||||
| Right | 0.512 | 0.023 | 0.517 | 0.025 | 0.507 | 0.024 | 2.35 | 2 | 0.10 | ||||||
| Cingulum (cingulate gyrus) | Left | 0.633 | 0.039 | 0.655 | 0.034 | 0.642 | 0.044 | 3.80 | 2 | 0.02 | |||||
| Right | 0.612 | 0.036 | 0.621 | 0.040 | 0.602 | 0.046 | 3.11 | 2 | 0.04 | ||||||
| Cingulum (hippocampus) | Left | 0.583 | 0.061 | 0.606 | 0.051 | 0.579 | 0.059 | 3.29 | 2 | 0.04 | |||||
| Right | 0.627 | 0.065 | 0.645 | 0.066 | 0.615 | 0.057 | 3.03 | 2 | 0.05 | ||||||
| CST | Left | 0.631 | 0.020 | 0.639 | 0.021 | 0.631 | 0.028 | 2.38 | 2 | 0.10 | |||||
| Right | 0.653 | 0.025 | 0.660 | 0.028 | 0.655 | 0.027 | 0.92 | 2 | 0.40 | ||||||
| Forceps major | 0.750 | 0.030 | 0.755 | 0.024 | 0.751 | 0.035 | 0.50 | 2 | 0.61 | ||||||
| Forceps minor | 0.571 | 0.023 | 0.587 | 0.028 | 0.569 | 0.038 | 6.56 | 2 | 0.002* | TC-HC | 0.02 | 0.005; 0.029 | 0.002 | 0.53 | |
| PTSD-HC | 0.002 | −0.01; 0.01 | 0.92 | 0.06 | |||||||||||
| TC-PTSD | 0.02 | 0.002; 0.028 | 0.02 | 0.62 | |||||||||||
| IFOF | Left | 0.537 | 0.026 | 0.549 | 0.028 | 0.538 | 0.038 | 2.39 | 2 | 0.10 | |||||
| Right | 0.537 | 0.028 | 0.544 | 0.029 | 0.538 | 0.034 | 0.68 | 2 | 0.51 | ||||||
| ILF | Left | 0.511 | 0.020 | 0.521 | 0.028 | 0.506 | 0.038 | 4.50 | 2 | 0.01 | |||||
| Right | 0.541 | 0.027 | 0.553 | 0.031 | 0.539 | 0.037 | 3.36 | 2 | 0.04 | ||||||
| SLF (parietal) | Left | 0.493 | 0.026 | 0.509 | 0.032 | 0.501 | 0.036 | 2.94 | 2 | 0.06 | |||||
| Right | 0.540 | 0.033 | 0.553 | 0.031 | 0.542 | 0.040 | 1.97 | 2 | 0.14 | ||||||
| SLF (temporal) | Left | 0.537 | 0.030 | 0.552 | 0.040 | 0.543 | 0.040 | 1.97 | 2 | 0.14 | |||||
| Right | 0.557 | 0.033 | 0.557 | 0.031 | 0.557 | 0.043 | 1.56 | 2 | 0.21 | ||||||
| Uncinate | Left | 0.529 | 0.040 | 0.526 | 0.034 | 0.530 | 0.037 | 0.18 | 2 | 0.84 | |||||
| Right | 0.632 | 0.045 | 0.621 | 0.045 | 0.630 | 0.046 | 0.77 | 2 | 0.46 | ||||||
3.3. Gray matter
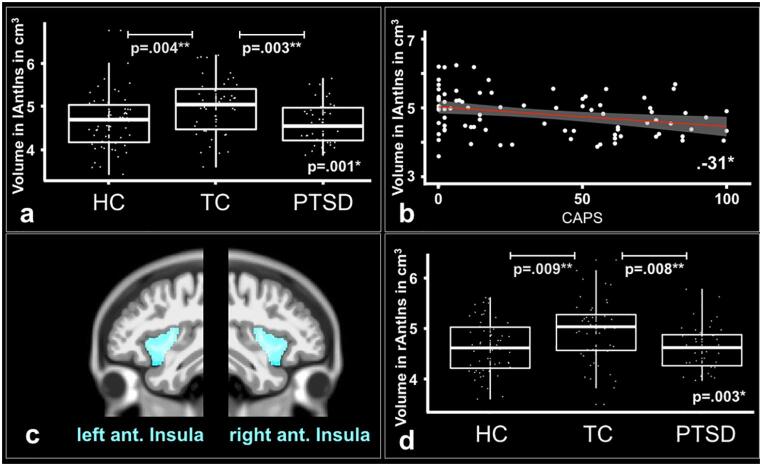
The RBM analysis revealed significant differences in GM volume (in cm3) in the left (F(2,140) = 7.24, p = .001; pbonf.cor. = 0.014) and right anterior insulae (F(2,140) = 6.06, p = .003; pbonf.cor. = 0.042; Fig. 2; Table 4). Post-hoc t-test comparisons showed that this difference in the left anterior insula was driven by the TC group, which showed a significantly higher mean GM volume than the HC subjects (MDifference = 0.35; 95% CI 0.09 to 0.60; p = .004; Hedges’ g = 0.54) or than the group of patients suffering from PTSD (MDifference = 0.39; 95% CI 0.12 to 0.66; p = .003; Hedges’ g = 0.67). The difference in the right anterior insula was also driven by the TC group, which showed a significantly higher mean GM volume than the HC subjects (MDifference = 0.30; 95% CI 0.06 to 0.54; p = .009; Hedges’ g = 0.50) or than the group of patients suffering from PTSD (MDifference = 0.33; 95% CI 0.08 to 0.59; p = .008; Hedges’ g = 0.60). There was no significant difference between patients with PTSD and HC. The gray matter dataset comprised 147 participants, excluding seven participants due to artefacts or missing data.
Fig. 2.
Region Based Morphometry. a) Boxplots with mean volume of left anterior insula (in cm3) in all three groups. The results show a significant ( αbonferroni_cor=.05/14 =.0036) difference in volume between patients with PTSD (n=42), TC (n=47) and HC (n=58) subjects. Post-hoc t-tests revealed significant differences in volume for the contrasts TC>HC subjects and TC>PTSD. b) Significant negative correlation ( αbonferroni_cor=.05/5 =.0125) between mean volume of left anterior insula (in cm3) and the mean CAPS score for TC subjects (n = 47) and patients with PTSD (n = 42). c) Outline of the left and right anterior insulae. d) Boxplots with mean volume of right anterior insula (in cm3) in all three groups. The results show a significant ( αbonferroni_cor=.05/14=.0036) difference in volume between patients with PTSD (n = 42), TC (n = 47) and HC (n = 58) subjects. Post-hoc t-tests revealed significant differences in volume for the contrasts TC>HC subjects and TC>PTSD.
[Abbreviations: CAPS - Clinician-Administered PTSD Scale; HC - Healthy control subjects; lAntIns - Left anterior insula; PTSD - Patients with posttraumatic stress disorder; rAntIns - Right anterior insula; TC - Trauma control subjects; * α <.05; ** α <.01; *** α <.001].
Table 4.
ROI comparisons of mean gray matter volume between all three experimental groups (patients with PTSD, TCs, HCs) with an ANCOVA including sex, age and total intracranial volume (TIV) as covariates.
*αbonferroni_cor=.05/14 =.0036;
[Abbreviations: HC – Healthy control subjects; PTSD – Subjects suffering from posttraumatic stress disorder; TC – Trauma control subjects]
|
Anatomical region |
Hemisphere |
Groups |
Analyses |
Hedgesg |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTSD N = 41 |
TC N = 47 |
HC N = 58 |
Fgroup |
df |
p |
contrast |
Difference |
CI [-95%; +95%] |
PTukey, HSD |
||||||
| M | SD | M | SD | M | SD | ||||||||||
| Hippocampus | Left | 3.18 | 0.29 | 3.32 | 0.35 | 3.18 | 0.33 | 3.37 | 2 | 0.04 | |||||
| Right | 3.52 | 0.33 | 3.61 | 0.39 | 3.50 | 0.38 | 1.50 | 2 | 0.23 | ||||||
| Amygdala | Left | 1.01 | 0.08 | 1.03 | 0.11 | 0.98 | 0.13 | 3.24 | 2 | 0.04 | |||||
| Right | 0.98 | 0.09 | 0.99 | 0.10 | 0.95 | 0.12 | 2.73 | 2 | 0.07 | ||||||
| Anterior Insula | Left | 4.63 | 0.49 | 5.02 | 0.65 | 4.67 | 0.64 | 7.24 | 2 | 0.001 | TC-HC | 0.35 | 0.09; 0.60 | 0.004 | 0.54 |
| HC-PTSD | 0.05 | −0.22; 0.31 | 0.92 | 0.07 | |||||||||||
| TC-PTSD | 0.39 | 0.12; 0.66 | 0.003 | 0.67 | |||||||||||
| Right | 4.63 | 0.44 | 4.96 | 0.63 | 4.66 | 0.58 | 6.06 | 2 | 0.003 | TC-HC | 0.30 | 0.06; 0.54 | 0.009 | 0.50 | |
| HC-PTSD | 0.03 | −0.21; 0.28 | 0.94 | 0.06 | |||||||||||
| TC-PTSD | 0.33 | 0.08; 0.59 | 0.008 | 0.60 | |||||||||||
| Posterior Insula | Left | 2.21 | 0.25 | 2.34 | 0.30 | 2.22 | 0.32 | 3.25 | 2 | 0.04 | |||||
| Right | 2.54 | 0.25 | 2.70 | 0.38 | 2.53 | 0.36 | 4.26 | 2 | 0.02 | ||||||
| Anterior Cingulate Gyrus | Left | 5.25 | 0.59 | 5.70 | 0.88 | 5.37 | 0.85 | 4.96 | 2 | 0.01 | |||||
| Right | 3.75 | 0.52 | 4.04 | 0.67 | 3.80 | 0.80 | 2.60 | 2 | 0.08 | ||||||
| Posterior Cingulate Gyrus | Left | 4.47 | 0.45 | 4.69 | 0.68 | 4.40 | 0.76 | 3.67 | 2 | 0.03 | |||||
| Right | 4.05 | 0.44 | 4.14 | 0.62 | 3.98 | 0.64 | 1.28 | 2 | 0.28 | ||||||
| Ventromedial Prefrontal Cortex | Left | 18.49 | 1.95 | 19.26 | 2.99 | 18.56 | 2.91 | 1.61 | 2 | 0.20 | |||||
| Right | 18.05 | 2.12 | 19.13 | 2.95 | 18.07 | 2.70 | 3.49 | 2 | 0.03 | ||||||
3.4. Relationship between white and gray matter differences
As a measurement of white- and gray matter coupling, we correlated findings of structural (WM and GM) differences between TC subjects and patients with PTSD. We found a significant positive correlation between the mean FA in the forceps minor and the mean volume in the left anterior insula including subjects from all three groups (r(1 4 4) = 0.29, 95% CI 0.14 to 0.43, p = .00036; pbonf.cor. = 0.001; Fig. 3a). The correlation stayed significant, when we included only patients with PTSD and TC subjects (r(86) = 0.31, 95% CI 0.10 to 0.49, p = .0037; pbonf.cor. = 0.015). Similarly, we found a significant positive correlation between the mean FA in the forceps minor and the mean volume in the right anterior insula (r(1 4 4) = 0.28, 95% CI 0.12 to 0.42, p = .0006; pbonf.cor. = 0.0024). This association also stayed significant, when we included only patients with PTSD and TC subjects (r(86) = 0.29, 95% CI 0.09 to 0.47, p = .0059; pbonf.cor. = 0.024; Fig. 3b).
Fig. 3.
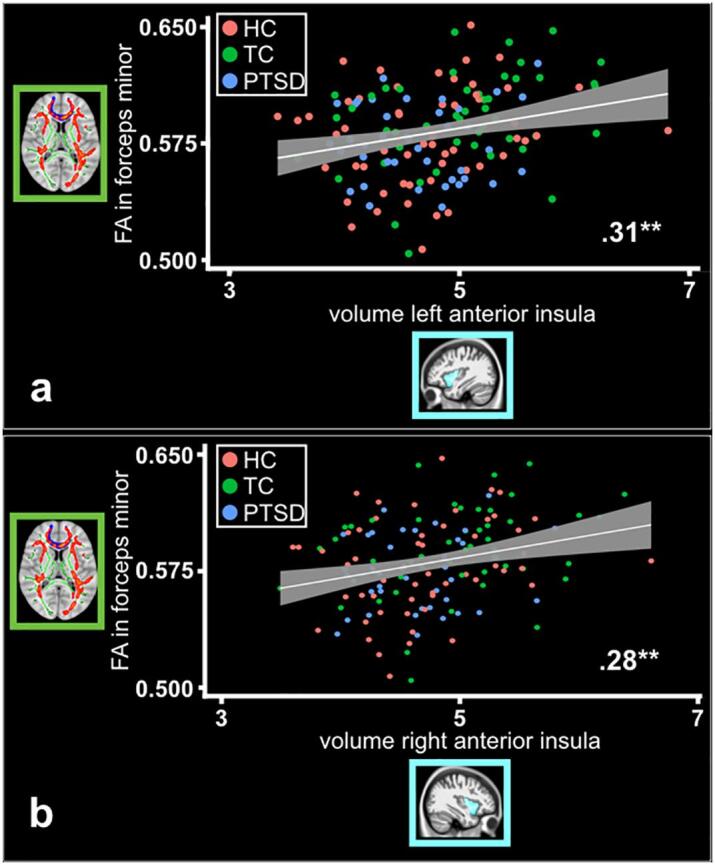
White and gray matter coupling. Significant positive correlation ( αbonferroni_cor =.05/4 =.0125) between mean FA value in forceps minor and a) volume of the lAntIns (PTSD, n = 42; TC, n = 47; HC, n = 58) and b) volume of the rAntIns (PTSD, n = 42; TC, n = 47; HC, n = 58).
[Abbreviations: HC - Healthy control subjects; lAntIns - Left anterior insula; PTSD - Patients with posttraumatic stress disorder; rAntIns - Right anterior insula; TC - Trauma control subjects; * α <.05; ** α <.01; *** α <.001].
3.5. Relationship of brain changes and clinical measures
We found significant negative Pearson correlation coefficients for the contrast of TC > PTSD between the mean FA value in the forceps minor and the mean CAPS- (r(87) = -0.32, 95% CI −0.12 to −0.49, p = .0026; pbonf.cor. = 0.008; Fig. 1c), STAIT- (r(84) = -0.26, 95% CI −0.05 to −0.45, p = .014; pbonf.cor. = 0.042) and ADS scores (r(83) = -0.28, 95% CI 0.07 to 0.46, p = .011; pbonf.cor. = 0.033). For the same contrast of TC > PTSD, we found a significant negative Pearson correlation coefficient between the mean GM volume in the left anterior insula and the mean CAPS score (r(82) = -0.31, 95% CI −0.10 to −0.49, p = .005; pbonf.cor. = 0.015). The correlations for the STAIT- (r(80) = -0.25, 95% CI −0.04 to −0.44, p = .023; pbonf.cor. = 0.069) and ADS score (r(78) = -0.24, 95% CI −0.02 to −0.43, p = .035; pbonf.cor. = 0.11) did not survive Bonferroni corrections. We did not find any significant correlations between our clinical measures and the GM volume difference in the right anterior insula.
4. Discussion
The present study used TBSS and ROI analysis for white matter and ROI analysis for gray matter regions to examine group differences in a large non-military sample of 154 patients with PTSD and trauma and healthy control subjects. We observed significant white- and gray matter differences in TC subjects compared to both patients with PTSD and HC subjects. In particular, TC subjects in comparison to patients with PTSD as well as HC subjects showed a significantly higher FA in the forceps minor and higher gray matter volume in the left and right anterior insulae. Interestingly, we did not find any differences in white or gray matter analyses between patients with PTSD and HC subjects. Our results suggest that TC subjects show higher interhemispheric frontal connections combined with larger volumes in brain areas associated with salience processing and threat detection than patients with PTSD and HC subjects. Furthermore, we found positive correlations between the FA value in the forceps minor and the volume of the left and right anterior insulae. These results suggest that higher volumes in the FM and anterior insulae in TC subjects might be a result of resilience, as TC subjects are those individuals that experienced a traumatic event, but did not develop PTSD. Finally, our results demonstrate a link between morphometric white and gray matter differences and symptom severity of PTSD, depression and trait anxiety. We argue that the forceps minor and the left anterior insula could be used as target regions in neuroscientifically-informed treatment studies on PTSD.
Our TBSS analysis revealed significantly higher FA values in TCs than patients with PTSD in long reaching white matter fibers such as the left CST and left IFOF. The CST is one of the largest descending white matter tracts in humans and involved in voluntary movement of contralateral limbs (Natali and Bordoni, 2018). Although, previous studies mention differences in FA in the CST in anxiety related disorders (Jenkins et al., 2016), depression (Sacchet et al., 2014) and neurogenerative disorders, such as Alzheimer’s (Douaud et al., 2011), its role in PTSD is unclear. Douaud et al. (2011) also found that higher FA values in the CST were associated with higher values in the SLF. Future studies are needed to determine the function of the CST in affect- and anxiety related disorders. The IFOF on the other hand originates in the parietal and occipital lobes and connects them with regions in the lateral frontal cortex (Catani et al., 2002). As a long reaching white matter tract, it is generally assumed to be involved in cognitive control, language processing (Almairac et al., 2015), and salience processing (Wang et al., 2016). Differences in the FA value of the IFOF have been previously linked to anxiety disorders in general (Jenkins et al., 2016) and PTSD in particular (Siehl et al., 2018). Furthermore, TC subjects showed a significantly higher FA value than HC subjects in the left IFOF, right ILF and right SLF. Similar to the IFOF, the SLF connects more posterior regions of the parietal, occipital and temporal lobe with the frontal lobe. The SLF is involved in a wide range of functions, such as the perception of visual and auditory space as well as aspects of motor behavior (de Schotten et al., 2011, Makris et al., 2005). In a previous systematic review, the authors did not find any differences in the SLF between TC and HC subjects (Siehl et al., 2018) and to the best of our knowledge, there is no study so far comparing the FA value in TC to HC subjects in a non-military sample. However, Our findings are in line with previous studies comparing patients with PTSD to TC subjects showing higher FA values in the SLF in TC subjects (Fani et al., 2012, Schuff et al., 2011). The ILF is a long reaching white matter tract connecting occipital and more posterior parts of the temporal lobe to more anterior parts of the temporal lobe. The ILF has been associated with visual cognition and socio-emotional processing of information (Herbet et al., 2018) and previously been associated to show reduced FA values in patients with PTSD in comparison to TC subjects (Olson et al., 2017). Interestingly we didn't find any differences in the ILF and SLF between TCs and patients with PTSD, but between TC and HC subjects. In a summary, long reaching white matter fibers connecting more posterior regions of the brain to more anterior regions seem to play a role in the development of PTSD. We speculate that lower white matter connectivity is an understudied factor in PTSD leading to higher salience and lower contextual information processing.
Frontal white matter tracts such as the FM might play an important role in the development of PTSD, in particular altered emotion regulation. Our white matter ROI analyses demonstrated significantly higher FA values in the FM in TC subjects than patients with PTSD or HC subjects. The FA in the FM was found to be central for PTSD in earlier studies (Sripada et al., 2012a). The forceps minor originates from the genu of the corpus callosum and connects the medial and lateral surfaces of the prefrontal cortices of both hemispheres in a fork-like shape, supporting interhemispheric information exchange between medial and lateral surfaces of the frontal lobe. Presumably, the FM is part of a larger network of white matter tracts, including the uncinate fasciculus and the cingulum, involved in emotion regulation (Versace et al., 2015). A lower FA in the FM suggests a lower top-down control and a less well orchestrated functional connectivity between hemispheres in the PFC (Liberzon and Abelson, 2016). Interestingly, the FA value in the FM in TC subjects was also significantly higher than in HC subjects, while there was no difference between patients with PTSD and HC subjects. White matter plasticity in the FM might occur after trauma experience as an adaptive change to strengthen networks involved in emotion regulation. However, longitudinal studies are needed to test this hypothesis. We did not find any significant FA differences between the groups in the uncinate fasciculus.
Lower gray matter volume in the insulae might be associated to weaker salience mapping in patients with PTSD. The RBM analysis on gray matter differences revealed a higher volume in the left and right insulae in TC subjects in comparison to both, patients with PTSD as well as HC subjects. The insula is a major hub within the salience network and associated with the detection and autonomic response to salient events as well the facilitation of communication between large scale networks (Menon, 2011, Menon and Uddin, 2010). A larger volumetric size of the insula can be associated with a stronger salience mapping. Stronger salience mapping after the experience of a traumatic event might lead to a better integration of cognitive, homeostatic and affective systems within the brain (Damasio and Carvalho, 2013, Pessoa, 2008). It also fits well to recent studies on neurofeedback, which found stronger functional connectivity between the PFC and the amygdala and insula after targeting areas in the salience network for up- or down regulation (Cohen Kadosh et al., 2016, Lubianiker et al., 2019, Paret et al., 2016). We did not find volumetric differences for the hippocampi, amygdalae, posterior insulae, anterior and posterior cingulate cortices or vmPFCs after Bonferroni corrections (see Table 4). We argue that this is partly due to low power and heterogeneity of the sample concerning the trauma type. Future studies should investigate volumetric gray matter differences with a larger sample focusing for example on one particular trauma type.
Structural differences in the FM and insulae are associated and point to the importance of the salience network in PTSD and its function in safety learning. We observed a positive association between the FA in the forceps minor and the volume of the left and right insulae (see Fig. 3), which supports the association between structural white and gray matter differences in patients with PTSD in comparison to TC subjects. This is also in line with previous studies emphasizing the central role of the insulae and the right amygdala within the salience network in PTSD (Cisler et al., 2014, Peterson et al., 2014, Rabinak et al., 2011, Sripada et al., 2012, Zhang et al., 2015), with the insulae playing a particular role in discrimination learning of safety cues (Lissek et al., 2014). The inclusion of two healthy control groups is important for the interpretation of the results at this point. The volumetric size of the left and right anterior insulae did not significantly differ between HCs and patients with PTSD. Due to our cross-sectional design, we can only speculate that this volumetric difference in the anterior insulae could emerge, post trauma, as an adaptive, functional neuroplastic change. The volumetric difference, alongside the difference in the FA of the FM, might lead to an increased functional connectivity in the salience network in TCs in comparison to patients with PTSD. This is in line with a recent study on healthy but highly trauma-exposed firefighters in comparison to non-firefighters, which found a higher functional connectivity in the salience network for the highly trauma exposed population of firefighters with the insula as a seed region (Jeong et al., 2018). Although it becomes more difficult to explain why there is no difference between patients with PTSD and HC subjects, it might also be possible that this difference existed before the traumatic event. Future studies on neurofeedback could investigate the effect of an up regulation of the anterior insulae in patients with PTSD in comparison to TC subjects and its effect on the functional connectivity within the salience network.
In our study, we included participants that experienced different types of traumatic events with the traumatic event either being caused voluntarily (e.g. physical violence, sexual abuse) or involuntarily (e.g. accidents, fire or explosion). Exposure to voluntarily caused events, in particular events involving interpersonal violence such as rape or sexual assault, show the strongest association with subsequent traumatic events (Benjet et al., 2016) and higher risk of developing PTSD (Kessler et al., 2017). Although our groups of patients with PTSD and TC subjects did not differ significantly in the type of trauma, patients with PTSD were more frequently exposed to voluntarily caused events, specifically physical violence and wartime experience. TC subjects on the other hand experienced more involuntarily caused events such as accidents. Whereas changes in white (Daniels et al., 2013, Daniels et al., 2013, Giedd and Rapoport, 2010) and gray matter (Bromis et al., 2018, Kribakaran et al., 2020) due to traumatic experiences were found to be age sensitive, it is not clear whether the different types of traumatic events impact white and gray matter trajectory differently. One would assume given the large differences in cognition, emotion and perception following either a voluntarily or involuntarily caused event. Future research is needed here to further assess differences in white and gray matter structures in adults with PTSD and trauma in adulthood with different types of trauma experiences.
These structural white and gray matter differences in areas related to salience processing and top-down control could inform behavioral prevention and treatment strategies. Psychotherapeutic interventions could benefit from neuroscientific findings by specifically selecting treatment techniques that focus on the flexibility (up- and down regulation) of salience processing and salience mapping, such as mindfulness-based interventions (Lanius et al., 2015), to increase the connectivity between the salience network and the frontal lobe in patients with PTSD. Higher emotional control, possibly mediated via the FM in combination with a threat-detection system that is well embedded might facilitate healthy recovery after the exposure to traumatic events.
4.1. Limitations
A limitation of this study is clearly its cross-sectional design, which only allows limited interpretation of the results. A longitudinal design would be needed to disentangle, if structural differences, especially between patients with PTSD and TC subjects, occur due to pre-existing vulnerabilities or if these differences have developed after trauma experience. Furthermore, our sample focused on adults with trauma experience in adulthood (after 18 years of age) only. White and gray matter are known to develop differently in underage populations suffering from PTSD in comparison to adults with PTSD, so we can draw only limited conclusions for this population from our sample. Further, the patients showed higher intake of medication and less years spending in education than both control groups. The differences in education might partly be explained by the experience of the traumatic event which on average participants in the PTSD and TC group experienced in their early to late twenties. Arguably, patients suffering of PTSD couldn’t continue their education due to the illness. Another possible argument could be the socio-economic background of participants which might have influenced the differences in years of education. This was however not assessed in our sample. While there was no difference between TC and HC control group in medication and education, we can’t fully rule out that group differences between patients with PTSD and both control groups are confounded.
4.2. Conclusions
In this cross-sectional study, we found structural white and gray matter differences in brain regions related to emotional control and threat detection in healthy traumatized control subjects in comparison to patients suffering from PTSD and HC subjects. First, TCs in comparison to patients with PTSD and HCs showed a higher FA in the forceps minor and a larger volume in the left and right anterior insulae. We argue that these morphometric differences might be associated with stronger emotion regulation and salience mapping in TC subjects. Second, we found a positive correlation between FA in the FM and gray matter volume in the insulae, showing that white and gray matter differences are associated and important for understanding the development of PTSD. Finally, the mean FA value in the forceps minor correlated negatively with symptom severity of PTSD, depression as well as trait anxiety, while gray matter volume in the left anterior insula correlated negatively with symptom severity in PTSD. Our results add important information for individualized prevention and neuroscientifically-informed treatment interventions such as neurofeedback, which could target the anterior insulae as a region to be up-regulated in patients with PTSD to strengthen functional connectivity within the salience network and between the salience network and regions in the frontal lobe. Finally, future studies could investigate long-term differences in the forceps minor before and after an intervention.
CRediT authorship contribution statement
Sebastian Siehl: Conceptualization, Investigation, Methodology, Software, Formal analysis, Writing - original draft, Visualization. Manon Wicking: Investigation, Writing - review & editing. Sebastian Pohlack: Investigation, Writing - review & editing. Tobias Winkelmann: Investigation, Writing - review & editing. Francesca Zidda: Investigation, Writing - review & editing. Frauke Steiger-White: Investigation. John King: Supervision, Writing - review & editing. Neil Burgess: Supervision. Herta Flor: Project administration, Resources, Supervision, Funding acquisition, Writing - review & editing. Frauke Nees: Conceptualization, Project administration, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank Birgül Sarun and Claudia Stief for help in data acquisition.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (SFB636/C1 to H.F. and NE 1383/14-1 to F.N.).
Data availability statement
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Ethical restrictions to protect participant confidentiality prevent us from making anonymised study data, publicly available. This also refers to the analysis/experimental code, and any other digital materials, where participant-related anonymised information (like sex or psychopathological status) are also included. Readers seeking access to the study data and materials should contact the corresponding author based on a formal collaboration agreement. This formal collaboration agreement indicates that data will be shared with other researchers who agree to work with the authors, and for the sole purpose of verifying the claims in the paper. The data and materials will be released to requestors after approval of this formal collaboration agreement by the local Ethics Committee of the Medical Faculty Mannheim.
Contributor Information
Sebastian Siehl, Email: sebastian.siehl@zi-mannheim.de.
Frauke Nees, Email: nees@med-psych.uni-kiel.de.
References
- Almairac F., Herbet G., Moritz-Gasser S., de Champfleur N.M., Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct. Funct. 2015;220(4):1983–1995. doi: 10.1007/s00429-014-0773-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (Vol. 1). https://doi.org/10.1176/appi.books.9780890423349.
- American Psychiatric Association. (2013). Guía de consulta de los criterios diagnósticos del DSM-5®. https://doi.org/10.1176/appi.books.9780890425657.
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Woolrich M.W., Jenkinson M., Johansen-Berg H., Nunes R.G., Clare S., Smith S.M. Characterization and Propagation of Uncertainty in Diffusion-Weighted MR Imaging. Magn. Reson. Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Benjet C., Bromet E., Karam E.G., Kessler R.C., McLaughlin K.A., Ruscio A.M., Koenen K.C. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med. 2016;46(2):327–343. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8(1):75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- Brewin C.R., Gregory J.D., Lipton M., Burgess N. Intrusive images in psychological disorders: characteristics, neural mechanisms, and treatment implications. Psychol. Rev. 2010;117(1):210–232. doi: 10.1037/a0018113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromis K., Calem M., Reinders A.A.T.S., Williams S.C.R., Kempton M.J. Meta-Analysis of 89 Structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. Am. J. Psychiatry. 2018;175(10):989–998. doi: 10.1176/appi.ajp.2018.17111199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in Vivo Interactive Dissection of White Matter Fasciculi in the Human Brain. NeuroImage. 2002;17(1):77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Steele J.S., Lenow J.K., Smitherman S., Everett B., Messias E., Kilts C.D. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: An exploratory fMRI study. J. Psychiatr. Res. 2014;48(1):47–55. doi: 10.1016/j.jpsychires.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Kadosh K., Luo Q., de Burca C., Sokunbi M.O., Feng J., Linden D.E.J., Lau J.Y.F. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. NeuroImage. 2016;125:616–626. doi: 10.1016/j.neuroimage.2015.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A., Carvalho G.B. The nature of feelings: evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013;14(2):143–152. doi: 10.1038/nrn3403. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.-P., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depression Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.-P., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma - A systematic review and meta-analysis (PSYNDEXshort) Depression Anxiety. 2013;30(3):207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- de Schotten M.T., Dell’Acqua F., Forkel S.J., Simmons A., Vergani F., Murphy D.G.M., Catani M. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011;14(10):1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- Douaud G., Jbabdi S., Behrens T.E.J., Menke R.A., Gass A., Monsch A.U., Smith S. DTI measures in crossing-fibre areas: Increased diffusion anisotropy reveals early white matter alteration in MCI and mild Alzheimer’s disease. NeuroImage. 2011;55(3):880–890. doi: 10.1016/j.neuroimage.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N., King T.Z., Jovanovic T., Glover E.M., Bradley B., Choi K., Ressler K.J. White Matter Integrity in Highly Traumatized Adults With and Without Post-Traumatic Stress Disorder. Neuropsychopharmacology. 2012;37(12):2740–2746. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa, E.B., 1995. PDS (Posttraumatic Stress Diagnostic Scale) Manual. In: Minneapolis: National Computer Systems.
- Foa E.B., Cashman L., Jaycox L., Perry K. The validation of a self-report measure of posttraumatic stress disorder: The posttraumatic diagnostic scale. Psychol. Assess. 1997;9(4):445–451. doi: 10.1037/1040-3590.9.4.445. [DOI] [Google Scholar]
- Fydrich, T., Renneberg, B., Schmitz, B., Wittchen, H., 1997. Strukturiertes Klinisches Interview für DSM-IV Achse II: Persönlichkeitsstörungen (SKID-II) [Structured clinical interview for DSM-IV. Axis II: Personality disorders]. In Göttingen: Hogrefe.
- Gaser C., Dahnke R. CAT-a computational anatomy toolbox for the analysis of structural MRI data. HBM. 2016:336–348. http://www.neuro.uni-jena.de/hbm2016/GaserHBM2016.pdf Retrieved from. [Google Scholar]
- Giedd J.N., Rapoport J.L. Structural MRI of Pediatric Brain Development: What Have We Learned and Where Are We Going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg K., Ein-Dor T., Solomon Z. Comorbidity of posttraumatic stress disorder, anxiety and depression: A 20-year longitudinal study of war veterans. J. Affect. Disord. 2010;123(1–3):249–257. doi: 10.1016/j.jad.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M. General Depression-Scale. ADS. Hogrefe; Göttingen: 1993. [Google Scholar]
- Herbet G., Zemmoura I., Duffau H. September 19). Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front. Neuroanat. 2018;12:77. doi: 10.3389/fnana.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins L.M., Barba A., Campbell M., Lamar M., Shankman S.A., Leow A.D., Langenecker S.A. Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. NeuroImage. Clinical. 2016;12:1022–1034. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H., Park S., Dager S.R., Lim S.M., Lee S.L., Hong H., Lyoo I.K. Altered functional connectivity in the fear network of firefighters with repeated traumatic stress. The British Journal of Psychiatry. 2018;1–7 doi: 10.1192/bjp.2018.260. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G., Koenen K.C. Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology. 2017;8(sup5):1353383. doi: 10.1080/20008198.2017.1353383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kribakaran S., Danese A., Bromis K., Kempton M.J., Gee D.G. Meta-analysis of Structural Magnetic Resonance Imaging Studies in Pediatric Posttraumatic Stress Disorder and Comparison With Related Conditions. Biol. Psychiatry: Cognit. Neurosci. Neuroimaging. 2020;5(1):23–34. doi: 10.1016/j.bpsc.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S., Gleich T., Lorenz R.C., Lindenberger U., Gallinat J. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol. Psychiatry. 2014;19(2):265–271. doi: 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- Kühn S., Gallinat J. Gray matter correlates of posttraumatic stress disorder: A quantitative meta-analysis. Biol. Psychiatry. 2013;73(1):70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Frewen P.A., Tursich M., Jetly R., McKinnon M.C. Restoring large-scale brain networks in ptsd and related disorders: A proposal for neuroscientifically-informed treatment interventions. Eur. J. Psychotraumatol. 2015;6:1–12. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux, L., Glanzmann, P., Schaffner, P. and Spielberger, C.D., 1981. Das state-trait-angstinventar [The state-trait anxiety inventory]. Hogrefe, Göttingen (in German).
- Liberzon I., Abelson J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron. 2016;92(1):14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Bradford D.E., Alvarez R.P., Burton P., Espensen-Sturges T., Reynolds R.C., Grillon C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc. Cognit. Affect. Neurosci. 2014;9(8):1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M., Bodammer N.C., Kühn S., Kaufmann J., Schütze H., Tempelmann C., Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48(13):3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Lubianiker N., Goldway N., Fruchtman-Steinbok T., Paret C., Keynan J.N., Singer N., Hendler T. Process-based framework for precise neuromodulation. Nat. Hum. Behav. 2019;3(5):436–445. doi: 10.1038/s41562-019-0573-y. [DOI] [PubMed] [Google Scholar]
- Makris N., Kennedy D.N., McInerney S., Sorensen A.G., Wang R., Caviness V.S., Pandya D.N. Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15(6):854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cognit. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S., Wakana, S., Van Zijl, P.C., Nagae-Poetscher, L., 2005. MRI Atlas of Human White Matter - Susumu Mori, S. Wakana, Peter C M van Zijl, L.M. Nagae-Poetscher - Google Books. Retrieved from https://books.google.de/books?hl=en&lr=&id=ltwRYlvFNLIC&oi=fnd&pg=PR5&dq=MRI+Atlas+of+the+Human+White+Matter&ots=gdMJnfbSmh&sig=Z8LsiJw0q8sxItglUdjKsQVrht4&redir_esc=y#v=onepage&q=MRI Atlas of the Human White Matter&f=false.
- Natali, A.L., Bordoni, B., 2018. Neuroanatomy, Corticospinal Cord Tract. In StatPearls. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/30571044. [PubMed]
- Olson E.A., Cui J., Fukunaga R., Nickerson L.D., Rauch S.L., Rosso I.M. Disruption of white matter structural integrity and connectivity in posttraumatic stress disorder: A tbss and tractography study. Depression Anxiety. 2017 doi: 10.1002/da.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paret C., Kluetsch R., Zaehringer J., Ruf M., Demirakca T., Bohus M., Schmahl C. Alterations of amygdala-prefrontal connectivity with real-time fMRI neurofeedback in BPD patients. Soc. Cognit. Affect. Neurosci. 2016;11(6):952–960. doi: 10.1093/scan/nsw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nat. Rev. Neurosci. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Peterson A., Thome J., Frewen P., Lanius R.A. Resting-state neuroimaging studies: A new way of identifying differences and similarities among the anxiety disorders? Can. J. Psychiatry. 2014;59(6):294–300. doi: 10.1177/070674371405900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak C.A., Angstadt M., Welsh R.C., Kenndy A.E., Lyubkin M., Martis B., Luan Phan K. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Frontiers. Psychiatry. 2011;2(NOV):1–8. doi: 10.3389/fpsyt.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchet M.D., Prasad G., Foland-Ross L.C., Joshi S.H., Hamilton J., Thompson P.M., Gotlib I.H. Structural abnormality of the corticospinal tract in major depressive disorder. Biol. Mood Anxiety Disord. 2014;4(1):8. doi: 10.1186/2045-5380-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio-Baptista C., Johansen-Berg H. White matter plasticity in the adult brain. Neuron. 2017;96(6):1239–1251. doi: 10.1016/j.neuron.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder U., Moergeli H. German version of clinician-administered PTSD scale. J. Trauma. Stress. 2002;15(6):487–492. doi: 10.1023/A:1020922023090. [DOI] [PubMed] [Google Scholar]
- Scholz J., Klein M.C., Behrens T.E.J., Johansen-Berg H. Training induces changes in white-matter architecture. Nat. Neurosci. 2009;12(11):1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N., Zhang Y., Zhan W., Lenoci M., Ching C., Boreta L., Neylan T.C. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. NeuroImage. 2011;54(Suppl 1):S62–S68. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl S., King J.A., Burgess N., Flor H., Nees F. Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta-analysis. NeuroImage: Clinical. 2018;19(May):581–598. doi: 10.1016/j.nicl.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl S., King J.A., Burgess N., Flor H., Nees F. Structural white matter changes in adults and children with posttraumatic stress disorder: A systematic review and meta-analysis. NeuroImage: Clin. 2018;19:581–598. doi: 10.1016/j.nicl.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Behrens T.E.J. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: Evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural Dysregulation in Posttraumatic Stress Disorder. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang Z., Ding W., Wan J., Zhuang Z., Zhang Y., Xu J. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0083473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R. R: A language and environment for statistical computing. 2013. ftp://ftp.uvigo.es/CRAN/web/packages/dplR/vignettes/intro-dplR.pdf Retrieved from.
- Versace A., Acuff H., Bertocci M.A., Bebko G., Almeida J.R.C., Perlman S.B., Phillips M.L. Dysregulation Disorders : a Probabilistic Tractographic Study. J. Am. Med. Assoc. Psychiatry. 2015;72(4):367–376. doi: 10.1001/jamapsychiatry.2014.2170.White. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Ji F., Hong Z., Poh J.S., Krishnan R., Lee J., Zhou J. Disrupted salience network functional connectivity and white-matter microstructure in persons at risk for psychosis: findings from the LYRIKS study. Psychol. Med. 2016;46(13):2771–2783. doi: 10.1017/S0033291716001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Young K.M. White matter plasticity in adulthood. Neuroscience. 2014;276:148–160. doi: 10.1016/j.neuroscience.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Wicking M., Steiger F., Nees F., Diener S.J., Grimm O., Ruttorf M., Flor H. Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol. Learn. Mem. 2016;136:116–126. doi: 10.1016/j.nlm.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.U., Wunderlich, U., Gruschwitz, S., Zaudig, M., 1997. SKID-I: Strukturiertes klinisches Interview für DSM-IV, Achse I: Psychische Störungen. [Structured clinical interview for DSM-IV. Axis I: Mental disorders]. Göttingen: Hogrefe.
- Zatorre R.J., Fields R.D., Johansen-Berg H. Plasticity in gray and white: Neuroimaging changes in brain structure during learning. Nat. Neurosci. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu F., Chen H., Li M., Duan X., Xie B., Chen H. Intranetwork and internetwork functional connectivity alterations in post-traumatic stress disorder. J. Affect. Disord. 2015;187:114–121. doi: 10.1016/j.jad.2015.08.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. Ethical restrictions to protect participant confidentiality prevent us from making anonymised study data, publicly available. This also refers to the analysis/experimental code, and any other digital materials, where participant-related anonymised information (like sex or psychopathological status) are also included. Readers seeking access to the study data and materials should contact the corresponding author based on a formal collaboration agreement. This formal collaboration agreement indicates that data will be shared with other researchers who agree to work with the authors, and for the sole purpose of verifying the claims in the paper. The data and materials will be released to requestors after approval of this formal collaboration agreement by the local Ethics Committee of the Medical Faculty Mannheim.