Abstract
A novel cold-adapted methane-oxidizing bacterium, termed TFB, was isolated from the thermoglacial Arctic karst spring, Trollosen, located in the South Spitsbergen National Park (Norway). The source water is cold and extremely low in phosphate and nitrate. The isolate belongs to the Methylovulum genus of gammaproteobacterial methanotrophs, with the closest phylogenetic affiliation with Methylovulum miyakonense and Methylovulum psychrotolerans (96.2 and 96.1% 16S rRNA gene sequence similarities, respectively). TFB is a strict aerobe that only grows in the presence of methane or methanol. It fixes atmospheric nitrogen and contains Type I intracellular membranes. The growth temperature range was 2–22°C, with an optimum at 13–18°C. The functional genes pmoA, mxaF, and nifH were identified by PCR, whereas mmoX and cbbL were not. C16:1ω5c was identified as the major fatty acid constituent, at an amount (>49%) not previously found in any methanotrophs, and is likely to play a major role in cold adaptation. Strain TFB may be regarded as a new psychrotolerant or psychrophilic species within the genus Methylovulum. The recovery of this cold-adapted bacterium from a neutral Arctic thermal spring increases our knowledge of the diversity and adaptation of extremophilic gammaproteobacterial methanotrophs in the candidate family “Methylomonadaceae”.
Keywords: arctic spring, cold adapted, fatty acids, methanotrophs, pMMO
Aerobic methane oxidation is performed by a unique group of methylotrophic bacteria found in various methane-containing environments, e.g. soil, lake sediments, rice paddies, landfills, tundra, and thermal and cold ecosystems (Hanson and Hanson, 1996; Dunfield et al., 2007), in which they play an important role in controlling the release of methane into the atmosphere (Conrad, 1996; Reeburgh, 2007). Most methane in nature is formed through anaerobic microbial processes by methanogenic archaea. However, thermogenic methane, a non-microbial source, is generated through the abiotic decomposition of buried organic materials and geochemical processes. This geological methane is freed to the atmosphere via seeps, mud volcanoes, gas venting, and geothermal areas (Etiope and Klusman, 2002; Fiebig et al., 2007; Nazaries et al., 2013).
Methane-oxidizing bacteria (MOB), or methanotrophs, possess highly specialized metabolism and have the ability to grow using only methane and a few other one-carbon compounds as their source of carbon and energy. These microorganisms currently belong to the phyla Proteobacteria, Verrucomicrobia, and Methylomirabilaeota (the candidate phylum NC10) (Houghton et al., 2019). A reclassification of the phylum Proteobacteria has more recently been recommended, which includes twenty-seven genera of proteobacterial methanotrophs (five genera of the class Alphaproteobacteria and twenty-two genera of the class Gammaproteobacteria) (Rahalkar et al., 2007; Trotsenko and Murrell, 2008; Deutzmann et al., 2014; Hirayama et al., 2014; Khalifa et al., 2015; Tavormina et al., 2015), and two genera of the phylum Verrucomicrobia were also suggested (Op Den Camp et al., 2009; Sharp et al., 2014; Van Teeseling et al., 2014). The taxonomic structure at the family level, within the order Methylococcales of gammaproteobacterial methanotrophs, was reconstructed using genome-based phylogeny, and three family lineages were proposed as Methylomonadaceae (Type Ia) (Parks et al., 2018), Methylococcaceae (Type Ib) (Orata et al., 2018), and Methylothermaceae (Type Ic) (Hirayama et al., 2014).
Several key functional genes, pmoA (encoding a subunit of the particulate methane monooxygenase: pMMO), mmoX (encoding a subunit of the soluble methane monooxygenase: sMMO), and mxaF (encoding the large subunit of methanol dehydrogenase: MDH), have been used to identify aerobic gammaproteobacterial methanotrophs from various environments. The pmoA gene is often used as a phylogenetic marker for the detection of aerobic methanotrophs in distinct habitats, including cold methane seeps in the floodplains of west Siberian rivers (Knief, 2015; Oshkin et al., 2016). The alphaproteobacterial genera (Type IIb) Methylocella (Dedysh et al., 2004) and Methyloferula (Vorobev et al., 2011) were described as psychrotolerant and acidophilic methanotrophs. They were isolated from cold ecosystems (wetlands) and only contain sMMO enzyme systems.
Methanotrophs generally occur in relatively cold environments, e.g. tundra soil, ground waters, arctic wetland soil, saline meromictic lakes, polar lakes, permafrost, and marine sediments (Bowman et al., 1997; Trotsenko and Khmelenina, 2005; Wartiainen et al., 2006). Until now, only two true psychrophilic species (optimal growth temperatures between 3.5 and 13°C), Methylosphaera hansonii (Bowman et al., 1997) and Methylobacter psychrophilus (Omelchenko et al., 1996), have been described and isolated from the surface sediments of an Antarctic meromictic lake and Russian Arctic tundra soil, respectively. Msp. hansonii requires saltwater for growth, whereas Mbc. psychrophilus does not need NaCl for growth. Several psychrotolerant Type Ia methanotrophs from various permanently cold habitats have been reported as new genera and species, including Methylovulum psychrotolerans (Oshkin et al., 2016), Methyloglobulus morosus (Deutzmann et al., 2014), Methylovulum miyakonense (Iguchi et al., 2010), Methyloprofundus sedimenti WF1T (Tavormina et al., 2015), Methylosoma difficile (Rahalkar et al., 2007), Methylobacter tundripaludum (Wartiainen et al., 2006), and Methylomonas scandinavica (Kalyuzhnaya et al., 1999). Three methanotrophic Type Ia bacteria, strain M200, Methylomonas strain M5 (Kip et al., 2011), and Methylomonas paludis strain MG30 (Danilova et al., 2013), were recently isolated from peat ecosystems. They were reported as being acid-tolerant (growth below a pH of 4.5). Furthermore, two sheathed and filamentous freshwater microorganisms, Crenothrix polyspora Cohn 1870 (Stoecker et al., 2006) and Clonothrix fusca Roze 1896 (Vigliotta et al., 2007), were identified and associated with gammaproteobacterial methane oxidizers. They appear to be psychrotolerant, but have not yet been grown in pure cultures.
Spitsbergen is the largest island of the high Arctic Svalbard archipelago, located north of Norway. It has a geologically active past and hot groundwater and glacial meltwater is mixed in several places, leading to karst springs at which the water stays above freezing temperature throughout the year (Lauritzen and Bottrell, 1994). One of the largest thermal springs on South Spitsbergen is Trollosen, located in the southernmost part (Fig. 1). Trollosen discharges water at a maximum rate of 18 m3 s–1, which varies with the seasons. Water temperature varies between 4 and 15°C. The smell of hydrogen sulphide (H2S) and the presence of organic slime in discharged water provides evidence of an active microbial community in the Trollosen aquifer (Lauritzen and Bottrell, 1994). In a previous study based on cultivation-independent analyses, sulphur-oxidizing chemolithotrophic Proteobacteria were found to dominate the bacterial community in this spring, with minor contributions by heterotrophs, including methane oxidizers (Reigstad et al., 2011). In the present study, water and slime samples were collected to investigate methane-utilizing bacteria in the Trollosen spring. A novel cold-adapted methanotrophic bacterium belonging to the candidate family “Methylomonadaceae” of the class Gammaproteobacteria was recovered.
Fig. 1.
Sampling location. (a) Map of South Spitsbergen National Park in Svalbard designating the sampling site (https://www.google.com/maps). (b) A close-up photograph of the Arctic thermal spring (Trollosen). The arrow shows the sampling location.
Materials and Methods
Sampling and growth conditions
In June 2006, approximately 40 mL of water, containing white slimy filaments, was collected from the thermal spring, Trollosen (76°72'N, 16°28'E), in the South Spitsbergen National Park during a joint expedition with another group from the University of Bergen (Reigstad et al., 2011). Samples were collected in Falcon tubes and stored at 8°C before being used as an inoculum for the enrichment and cultivation of methanotrophs. The in situ temperature and pH of the sample were 15°C and 6.8, respectively. The 3×1 m cave opening of the spring is dominated by glacial meltwater from the nearby Vitkovskij glacier and discharged approximately 8 m3 s–1 at the time of sampling. On the day of sampling, the water contained (in decreasing order of concentrations in mM); Cl–, 56; Na, 2.76; Ca, 2.46; Mg, 0.81; ammonia, 0.19; SO42–, 0.18; Li, 0.1; and lesser amounts of Al, Fe, B, Mn, As, Mo, and Ba. The amounts of nitrate and phosphate were below the detection limit (Reigstad et al., 2011).
A previously described low-salt mineral medium (LMM) with pH 6.8 and vitamins was used for enrichment conditions (Islam et al., 2015), except that KNO3 was replaced with NH4Cl (0.1 g L–1). Thereafter, 2 mL of the water sample was added directly to 20 mL of LMM in a 120-mL serum bottle. The bottle was closed with butyl rubber caps and aluminium crimped seals. A mixture of methane (80%) (purity of methane, 99.5%; Yara Praxair), air (10%), and CO2 (10%) was added aseptically via a syringe into the headspace. The bottle was incubated at 18°C in the dark and without shaking for three weeks. The gas mixture was replaced every seven days. After the incubation, the enrichment culture became visibly turbid and was examined using a phase-contrast microscope (Eclipse E400 microscope; Nikon). Thereafter, 2 mL of the culture was transferred to fresh LMM and re-incubated under the same conditions as those described above.
Enrichment, isolation, and morphology
To recover a true methane oxidizer, the environmental enrichment sample was transferred five times into fresh LMM and incubated with methane, air, and CO2. Serial dilutions (10–6 to 10–8) were prepared and 0.1-mL aliquots were spread onto either gelrite (20 g L–1, GelzanTM CM; Sigma-Aldrich) or agar (Difco) plates containing LMM. The plates were then incubated for four weeks at 18°C in jars filled with a methane/air (2:1) mixture. Individual colonies were collected, streaked onto fresh plates, and re-incubated for three weeks. A single colony was then selected and examined by phase-contrast microscopy. The selected colony was transferred to fresh liquid LMM and incubated for three weeks under the same growth conditions. After a pure culture was extracted, LMM was used for its routine cultivation at 15 and 18°C for two weeks. The purity of the culture was assessed by phase-contrast and electron microscopies, observations of single colony growth on plates, and a heterotrophic contamination test using Luria-Bertani broth (1 to 5% [v/v]) and glucose (10 mM). In addition, growth was examined in the diluted and undiluted nitrate or ammonium mineral salt media used to cultivate the methanotrophs (Whittenbury et al., 1970). Morphology was assessed using a phase-contrast microscope and Jeol-1230 electron microscope. Exponentially-grown cells were harvested by centrifugation and used to prepare ultrathin sections for transmission election microscopy (TEM), as described previously (Islam et al., 2015).
Utilizable carbon and nitrogen sources, pH, temperature, and salt concentration
Various organic compounds were tested as possible carbon and energy sources at a concentration of 10 mM in fresh LMM. The following substrates were tested: glucose, acetate, pyruvate, lactate, malate, succinate, ethanol, sucrose, fructose, maltose, mannitol, sorbitol, Luria-Bertani broth, and yeast extract. Growth on methanol, methylamine, formate, and formaldehyde was tested at concentrations between 0.03% and 0.2% (v/v) in LMM. To prevent vaporization, bottles were capped with butyl-rubber stoppers. Growth was also tested in nitrogen-free LMM (without KNO3) and adjusted to pH 6.8 in triplicate, and the only nitrogen source was N2 from the air. After three weeks of incubation, resultant growth was evaluated. The growth temperature range was tested at 0, 2, 5, 10, 13, 15, 18, 20, 22, 25, and 30°C (at pH 6.8). The effects of pH on growth were investigated and the test for antibiotic sensitivity was performed as previously described (Islam et al., 2020). The effects of salt concentrations on growth were also assessed by adding NaCl (0.1, 0.5, 1, 2.0, and 3% [w/v]) to LMM. Growth was assessed after two weeks of incubation using optimal culture conditions.
Acetylene inhibition test, naphthalene assay, and fatty acid analysis
To assess the effects of acetylene, 4% of acetylene (v/v) was added into the headspace after five days of incubation with methane or methanol. Triplicate flasks were used for the methane oxidation inhibition test. One flask without acetylene was used as a control. The naphthalene-oxidation assay was performed as described previously (Islam et al., 2020). A phospholipid fatty acid (PLFA) analysis of cells grown at 15°C was performed at the DSMZ (Deutsche Sammlung von Mikroorganismen und Zelkulturen GmbH).
DNA isolation and 16S rRNA and functional gene analyses
Genomic DNA was isolated using the CTAB procedure (Murray and Thompson, 1980). The 16S rRNA gene was amplified as described previously (Islam et al., 2008) using a Peltier thermal cycler (PTC-200; MJ Research). PCR products were purified and sequenced using the BigDye kit for automated DNA sequencers (ABI 3700 PE; Applied Biosystems). Amplification of the functional genes pmoA, mmoX, mxaF, nifH (nitrogenase iron protein), and cbbL (the large subunit of the ribulose-1,5-bisphosphate carboxylase/oxygenase, RubisCO) was performed using genomic DNA (Table S1). PCR was accomplished using Dynazyme™ High-Fidelity DNA Polymerase (Finnzymes) and the PCR program was previously described (Islam et al., 2015). Methylococcus capsulatus (strain Bath) DNA was used as a positive control. MilliQ water was used as a negative control. The PCR products of the gene pmoA were cloned using pCR4-TOPO (Invitrogen) according to the manufacturer’s protocol. The four independent clones of the pmoA gene were sequenced and confirmed to be identical. The Southern blotting technique was utilized for the verification of pMMO and sMMO. DNA from Mcc. capsulatus (Bath) and Methylacidiphilum kamchatkense (Kam1) were used as positive and negative controls, respectively. Genomic DNA from the pure isolate was digested with EcoRI and HindIII. Fragments of DNA were separated using agarose gel electrophoresis and transferred onto Hybond-N nylon membranes (Amersham Biosciences). The probes utilized for hybridization were made by PCR using the pmoA and mmoX primer sets (Table S1) and labeled with [α-32P]dCTP using the DNA labeling kit (Amersham Biosciences) as previously described (Baxter et al., 2002; Islam et al., 2008).
Phylogenetic analysis and accession numbers
The 16S rRNA sequences and deduced protein sequences of the functional genes (using the ExPASy Translate tool; https://web.expasy.org/translate) were evaluated with available sequences in the GenBank database using BLASTn and BLASTp (the NCBI tools), respectively (Table S2). 16S rRNA gene and deduced amino acid (PmoA) sequences were aligned using the CLUSTAL W algorithm. Phylogenetic trees were constructed using different methods, such as the Neighbor-Joining, Maximum Likelihood, and Minimum-Evolution methods, based on the following models: Kimura 2-parameter, Maximum composite likelihood, Tamura 3-parameter, Jukes-Cantor, Jones Taylor-Thornton (JTT), Dayhoff, and Poisson. These models were implemented in the MEGA7 software package (Kumar et al., 2016). The sequences of the 16S rRNA, pmoA, mxaF, and nifH genes of strain TFB have been deposited in GenBank under accession numbers GQ130272, KX282487, KX282488, and KX282489, respectively.
Results and Discussion
Isolation of strain TFB and morphological characteristics
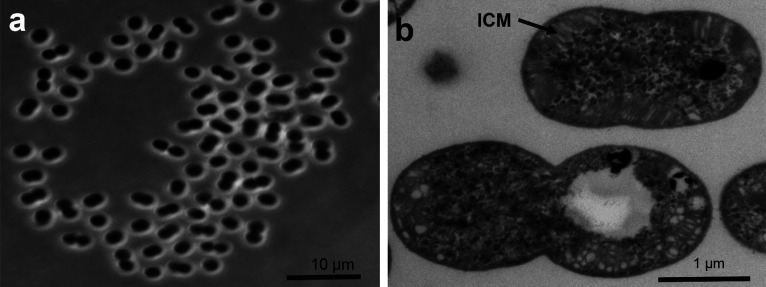
After three weeks of incubation at 18°C with methane, the enrichment was turbid, and phase-contrast microscopy revealed the presence of non-motile, straight, or curved rod-shaped cells, small cocci, and large coccoid cells with a thick mucous capsule. The large coccoid cells demonstrated poor growth with NH4Cl in enriched LMM. After five successive transfers into fresh LMM (with KNO3), the fraction of large coccoid cells was larger. After 3–4 weeks of incubation, the following colonies were observed on gelrite plates: transparent (approximately 1.4 to 1.8 mm in diameter), light yellow (approximately 0.5 to 1.0 mm), small white (0.8 to 1 mm), and large white (2.0 to 2.5 mm). Transparent colonies were not present on the agar plates (even after five weeks of incubation). However, small and large white colonies were observed. To detect a true methane oxidizer, three different colonies were transferred to fresh LMM separately and incubated with methane, air, and CO2. Only a single transparent colony from the gelrite plate showed growth in methane after two weeks of incubation, whereas no growth was observed for the white or light-yellow colonies. A true cold-adapted methanotrophic isolate was obtained from the transparent colonies on the gelrite plates. The isolate, called TFB, was a large coccoid/elliptical rod with a length of 1.5–2.0 μm and diameter of 1.2–1.5 μm. The strain grew in LMM with methane or methanol (0.2%). It did not grow in the presence of methane or methanol under anaerobic conditions or in the absence of methane or methanol. These cells were non-motile and occurred individually or in pairs. An investigation by electron microscopy revealed the presence of intracellular membranes (ICM) stacked (as vesicular disks) throughout TFB cells, which is a common feature of the methanotroph family Methylomonadaceae (Fig. 2). Spores or cysts were not observed in TFB using TEM. The isolate had a polysaccharide-like matrix around it, which was also observed in the acid-tolerant methanotrophic strain M200 (Kip et al., 2011). Similar bacteria, also described as large morphotype 2 psychrophilic methanotrophs, have been observed in enrichments from Russian Arctic tundra soil (Berestovskaya et al., 2002). Moreover, these large cocci, with thick mucous capsules, grew at a pH and temperature between 5–7 and 5–10°C, respectively.
Fig. 2.
Cell morphology of strain TFB. (a) Phase-contrast micrograph of cells grown in LMM, containing methane, for 7 days. (b) Transmission electron micrograph showing intracytoplasmic membrane (ICM) systems. Bars, 10 μm (a) and 1 μm (b).
Physiological characteristics
A comparison of the major characteristics of strain TFB with related cold-adapted (e.g. psychrophilic and psychrotolerant) and mesophilic Type Ia methanotrophs is shown in Table 1. Strain TFB utilized only methane and methanol as the sole carbon and energy sources, respectively. It did not grow on substrates containing multi-carbon compounds or in complex media, evincing obligate methanotrophy. Growth in methanol was observed at concentrations between 0.05 to 0.2% (v/v). Cells utilized nitrate and ammonium salts as nitrogen sources. In the absence of vitamins, TFB grew very poorly. The isolate grew on nitrogen-free LMM, demonstrating its ability to fix atmospheric N2, whereas growth was more optimal in LMM containing KNO3. Strain TFB grew between 2 and 22°C, with optimal growth occurring between 13 to 18°C (Fig. S1). This result implies that strain TFB is more psychrotolerant/psychrophilic than the acid-tolerant methanotrophic strain M200 and mesophilic Mvm. miyakonense HT12T. Although Mvm. psychrotolerans strains grew efficiently at temperatures lower than 10°C, their optimal growth temperature was 20–25°C (Oshkin et al., 2016). No growth was observed at 25°C, indicating that strain TFB is not a member of the mesophilic Type Ia methanotrophs. The specific growth rate (μ) and doubling time at the optimum temperature were 0.017 h–1 and 18 h, respectively. At 15°C, strain TFB grew at a pH interval of 5.2–8.5; however, optimal growth was observed at a pH 6.8–7.2. It did not show any growth at pH 5.0 or 9.0, demonstrating that strain TFB is a neutrophilic bacterium rather than a member of the acid-tolerant or alkaline methanotrophs. The strain did not require excess NaCl for growth and grew favorably in LMM with 0.1% NaCl (w/v). When NaCl concentrations exceeded 0.5% (w/v), growth was inhibited. Acetylene acts as a suicide substrate for methanotrophs containing pMMO (Prior and Dalton, 1985). The effects of acetylene (4%) on TFB showed that growth and methane oxidation were completely suppressed, which confirmed the presence of pMMO as the functional methane oxidization enzyme. This result is consistent with previous findings obtained on other Type Ia, Type Ib, and verrucomicrobial methanotrophs (Bedard and Knowles, 1989; Islam et al., 2008).
Table 1.
Comparisons of major characteristics of the cold-adapted strain TFB and Type Ia gammaproteobacterial methanotrophs. Strains: 1) Strain TFB (this study); 2) Methylovulum psychrotolerans (strains Sph1T, Sph2, and OZ2) (Oshkin et al., 2016); 3) Methanotrophic strain M200 (Kip et al., 2011); 4) Methylovulum miyakonense HT12T (Iguchi et al., 2010); 5) Methyloprofundus sedimenti WF1T (Tavormina et al., 2015); 6) Methylosoma difficile LC 2T (Rahalkar et al., 2007); 7) Methyloglobulus morosus KoM1T (Deutzmann et al., 2014); 8) Methylobacter tundripaludum SV96T (Wartiainen et al., 2006); and 9) Methylosphaera hansonii ACAM 549T (Bowman et al., 1997). +, positive results; –, negative results; nr., not reported.
| Characteristics | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Cell morphology | Cocci, elliptical rods |
Cocci | Cocci | Cocci or rods | Cocci, elliptical rods |
Cocci, elliptical rods |
Short rods | Rods | Cocci |
| pMMO | + | + | + | + | + | + | + | + | + |
| sMMO | – | – | – | + | – | – | – | – | – |
| mxaF gene | + | + | nr | + | + | + | + | + | nr |
| nifH gene/N2 fixation | + | + | + | + | + | + | + | + | + |
| Pigmentation | Transparent | Light pink | Pink | Pale brown | nr | Pale pink | Red pink | Pale pink | –d |
| Growth temp. (opt.) | 2–22 (13–18) | 2–36 (20–25) | 4–30 (nr) | 5–34 (24–32) | 4–26 (18–23) | 10–30 (25) | 4–30 (20) | 10–30 (23) | 0–21 (10–13) |
| pH range | 5.2–8.5 (6.5–7.2) | 4.0–8.9 (6.0–7.0) | 4.1–7.0 (5.5) | 6.0–7.5 | 6.0–8.0 (6.5–7.5) | 5–9 (6–8) | 5–8.5 (6–8) | 5.5–7.9 | 7.0 |
| Growth on methanol | + | + | + | + | + | + | + | –c | + |
| Vitamin required | + | – | – | – | + | + | – | – | –e |
| G+C content (mol%)a | nd | 51.4–51.9 | nr | 49.3 | 40.5 | 49.9 | 47.7 | 47 | 43–46 |
| G+C content (mol%)b | 52.2 | – | 52.2 | 53.4 | – | 52.7 | 54 | 50.7 | – |
| Source | Arctic thermal Spring |
Cold methane seeps and freshwater lake |
Acidic Sphagnum peat |
Forest soil | Marine sediment | Lake sediment | Lake sediment | Wetland soil | Antarctic meromictic lake |
aThe G+C content was assessed by HPLC (DSMZ; Mesbah et al., 1989; Kamagata and Mikami, 1991). b16S rRNA, pmoA, mxaF, and nifH sequences were used to measure the DNA G+C content (mol%). cShows poor to no growth on methanol. dHighly purified agar, agarose, and gelrite were likewise unsuccessful for pigmentation. eSeawater required.
Fatty acid profile
Strain TFB exhibited a unique fatty acid profile from the known psychrophilic and psychrotolerant methanotrophic genera of MOB (Table 2). The predominant fatty acids were C16:1ω5c, C14:0, and C16:1ω7c. The fatty acid C16:1ω5c is generally found in related psychrotolerant species, such as Mvm. psychrotolerans and the methanotrophic strain M200. The genera Methyloterricola oryzae 73aT (a member of Type Ib in the family Methylococcaceae) contained 28.3% of C16:1ω5c, whereas strain TFB contained 49.65%. This was relatively high, and such a high percentage of C16:1ω5c has not previously been reported in any gammaproteobacterial methanotrophs described to date. A recent study reported that Mvm. psychrotolerans adapts to decreasing growth temperatures by increasing unsaturation in bulk fatty acids, including C14 and C16, as well as in hopanoids (Bale et al., 2019). At 4°C, Mvm. psychrotolerans possesses 88–91% unsaturated fatty acids (mostly isomers of C16:1 and C14:1ω7), and 79–80% when grown at 20°C (Bale et al., 2019). The high amount of C16:1ω5c in strain TFB indicates that it plays an important role in cold adaptation and may be used as a diagnostic feature in identifying cold-adapted methanotrophs and differentiating them from other mesophilic or thermotolerant methanotrophs. The amounts of C14:0, C16:1ω7c, and C16:0 were similar to those in other psychrophilic, psychrotolerant, and mesophilic methanotrophic genera. Additionally, C16:1ω7c in strain TFB showed a significant difference from Mvm. myakonense strain HT12T, while acid-tolerant methanotrophic strain M200 contained the fatty acid C16:1ω8c (37.4%), which was completely absent in strain TFB.
Table 2.
Comparison of cellular fatty acid compositions of cold-adapted strain TFB with other psychrophilic, psychrotolerant, and mesophilic Type Ia and Type Ib methanotrophs. 1) Strain TFB (this study); 2) Methylovulum psychrotolerans (strains Sph1T, Sph2, and OZ2) (Oshkin et al., 2016); 3) Methanotrophic strain M200 (Kip et al., 2011); 4) Methylovulum miyakonense HT12T; 5) Methyloprofundus sedimenti WF1T (Tavormina et al., 2015); 6) Methylosoma difficile LC 2T (Rahalkar et al., 2007); 7) Methyloglobulus morosus Kom1T (Deutzmann et al., 2014); 8) Methylobacter tundripaludum SV96T (Wartiainen et al., 2006); 9) Methylosphaera hansonii ACAM 549T (Bowman et al., 1997); 10) Methyloterricola oryzae 73aT (Frindte et al., 2017); and 11) Methylococcaceae strains BRS-k6, GFS-K6, and AK-K6 (Islam et al., 2015). Values are given as a percentage of total fatty acids.
| Fatty acids | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | 0.96 | – | – | – | – | 2.74 | 0.1 | – | – | – | – |
| C13:1 | 1.53 | – | – | – | – | – | – | – | – | – | – |
| C14:0 | 18.28 | 7.1–9.3 | 7.5 | 34.2 | – | 8.55 | 0.7 | 34.9 | 2–3 | – | 3.73–8.43 |
| C15:0 | 1.22 | – | 0.7 | 2.97 | – | 0.79 | – | 23.4 | 1–2 | – | – |
| C16:1ω8c | – | 22.7–30.1 | 37.4 | – | 22.3 | – | 55.3 | 8.2 | 37–41 | – | – |
| C16:1ω7ca | 16.52 | 22.5–33.0 | 14.9 | – | 3– | 60.0 | 5.8 | – | 16–19 | 26.9 | 57.93–69.41 |
| C16:1ω6c | – | 5.7–6.2 | 9.4 | 6.40 | – | 15.0 | 28.7 | 26.3 | 17–18 | 8.7 | – |
| C16:1ω5c | 49.65 | 17.3–19.2 | 24.3 | – | – | – | – | – | – | 28.3 | 11.38–30.02 |
| C16:1ω7t | – | – | – | – | – | – | 6.8 | – | 2–3 | – | – |
| C16:1ω9t | – | – | – | 26.9 | – | – | – | – | – | – | |
| C16:0 | 9.18 | 6.2–11.4 | 4.1 | 46.9 | 15 | 8.5 | – | – | 14–15 | 30.9 | 4.72–11.37 |
| C16:1ω9c | – | – | – | – | 28.8 | – | – | – | – | – | – |
| C16:1ω10c | – | – | – | – | – | – | – | – | – | 2,4 | – |
| C16:1ω11c | – | – | – | – | – | 2.44 | – | – | – | – | – |
| C16:0 3OH | 2.66 | – | – | 8.00 | – | 1.31 | 1.0 | – | – | – | 1.50–1.54 |
| C16:2ω9,14 | – | – | – | 7.1 | – | – | – | – | – | – | |
| C18:1ω7c | – | 0.7–1.2 | – | – | – | – | 0.9 | – | 1–2 | 1.7 | – |
| C18:1ω9c | – | 0–1.1 | – | – | – | – | – | – | – | – | – |
| βOH-nC16:0 | – | 2.8–4.6 | – | – | – | – | – | – | – | – | – |
| Growth temp. (°C) | 15 | 20 | nr* | 24 | 23 | 25 | 20 | 20 | 10 | 30 | 25 |
* not reported
Functional genes and phylogenetic analyses
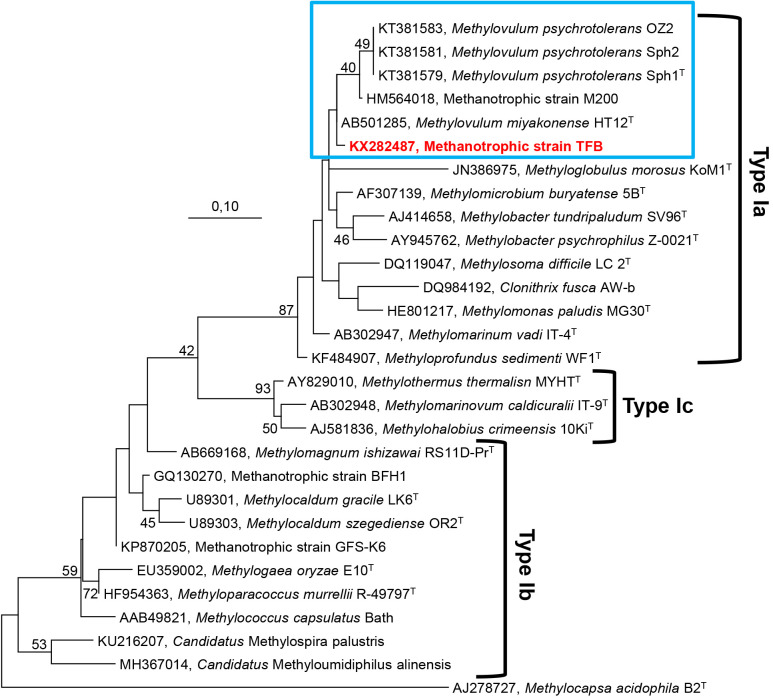
Partial fragments of the functional genes pmoA, mxaF, and nifH were amplified and sequenced. The results obtained indicated a close relationship to corresponding genes in other Type Ia methanotrophs. PCR amplification trials on mmoX and subsequent Southern blotting (Table S3) yielded negative results that substantiated the negative naphthalene assay, thereby confirming the lack of genes encoding for sMMO in strain TFB. Furthermore, no feasible PCR band for the cbbL gene was detected, revealing that the strain may not utilize genes encoding RuBisCO for autotrophic CO2 fixation. The presence of RuBisCO in certain Type Ib methanotrophs (such as Methylococcus, Methylocaldum, Methylomagnum, Methyloterricola, Methylospira, and methanotrophic strains BFH1, BFH2, and LS7-MC) has been reported (Baxter et al., 2002; Danilova et al., 2013; Islam et al., 2015; Islam et al., 2016; Frindte et al., 2017), whereas Type Ia methanotrophs, as well as some Type Ib methanotrophs (such as Methylomonas, Methylomicrobium, Methylomarinum, Methylotetracoccus, Methyloparacoccus, and Methylogaea), do not contain RuBisCO (Baxter et al., 2002; Hirayama et al., 2013; Ghashghavi et al., 2019). The nearly complete 16S rRNA gene (1411 bases) and PmoA (169 amino acids) sequences of the methanotrophic strain TFB were used to construct phylogenetic trees.
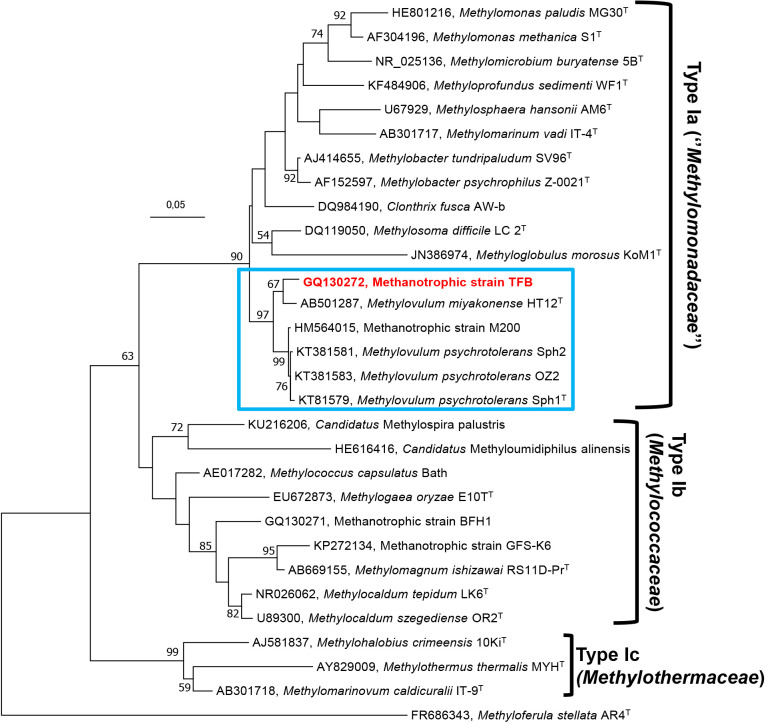
Members of psychrotolerant Type Ia methanotrophic strains, such as Msm. difficile LC 2T (Rahalkar et al., 2007), Mbc. tundripaludum SV96T (Wartiainen et al., 2006), and Mbc. psychrophilus Z-0021T (Omelchenko et al., 1996), have 16S rRNA gene sequence identities of 93.0–93.6% to strain TFB. According to further 16S rRNA gene analyses, the methanotrophic bacterium M200 (Kip et al., 2011), Mvm. psychrotolerans (strains Sph1T, Sph2, and OZ2), and Mvm. miyakonense HT12T (Iguchi et al., 2010) were identified as being closely related to strain TFB, with 96.6, 96.4–96.2, and 96.0% sequence similarities, respectively (Table S2). Furthermore, a phylogenetic analysis of the deduced amino acid sequence of pmoA showed high sequence similarities with strain M200 (98.8%), Mvm. psychrotolerans (98.8%), Mvm. miyakonense (100%), Mbc. tundripaludum (99.3%), Mbc. psychrophilus (97.5%), Msm. difficile (97.6%), and C. fusca (96.3%). The BLASTp search of MxaF protein sequences from the strain TFB showed the strongest associations with Mvm. miyakonense (96.6%), Mvm. psychrotolerans (96.6%), Mbc. tundripaludum (93.5%), and Mgb. morosus strain Kom1 (91.2%). NifH protein sequences showed associations with Mvm. miyakonense, Mvm. psychrotolerans, Mbc. tundripaludum, Methylocucumis oryzae (100%), and Mpf. sedimenti (95.2%). To establish the affiliation of two or more strains to the same genus, a minimum of 95% identity for 16S rRNA gene sequences is required (Schloss and Handelsman, 2004; Adekambi et al., 2008). 16S rRNA gene sequence similarities (Table S2) between strain TFB (Arctic thermal spring), strain M200 (Sphagnum mosses), and Mvm. miyakonense HT12T (forest soil) were 96.0–96.6%. This result suggests that strains TFB and M200 belong to the genus Methylovulum, or that they may denote two separate species, or even represent a new genus within Type Ia methanotrophs. Phylogenetic analyses of the 16S rRNA and pmoA genes of the strain TFB revealed that it, along with Methylovulum species (Mvm. miyakonense and Mvm. psychrotolerans) and the methanotrophic strain M200, most probably constitute a new cluster in the Type Ia methanotrophs of the class Gammaproteobacteria (Fig. 3, 4, S2, S3, S4, and S5). This phylogenetic assumption was facilitated using physiological characteristics (Table 1) and chemotaxonomic studies (Table 2). Furthermore, the 16S rRNA, pmoA, mxaF, and nifH sequences may contribute to the detection of related methane oxidizers from cold habitats and demonstrate how these cold-adapted bacteria are widely dispersed. Based on the 16S rRNA gene analysis, physiological properties, and fatty acid compositions, we propose that the colorless and neutrophilic methanotrophic strain TFB represents a new species of the genus Methylovulum. Strain TFB may be referred to as ‘Methylovulum sp. TFB’. However, the complete genus status of methanotrophic bacteria, namely Mvm. miyakonense HT12T, Mvm. psychrotolerans Sph1T, acid-tolerant methanotrophic strain M200, and the neutrophilic methanotrophic strain TFB require further investigations (e.g. comparisons including the whole genome) before decisions may be reached regarding the genus level.
Fig. 3.
Molecular phylogenetic analysis (16S rRNA gene sequences) using the Maximum Likelihood method based on the Kimura 2-parameter model of strain TFB (indicated in bold red) and other related Type Ia, Type Ib, and Type Ic gammaproteobacterial methanotrophs. Evolutionary analyses were conducted in MEGA7. Nodes supported by bootstrap values (percentages of 1,000 data resamplings) ≥50% are shown at each node. The Type IIb methanotroph, Methyloferula stellata AR4 (FR686343) of the class Alphaproteobacteria (in the family Beijerinkiaceae) was used as an outgroup. Bar, 0.05 substitutions per nucleotide.
Fig. 4.
Molecular phylogenetic analysis (deduced amino acid sequences of the pmoA gene) of strain TFB (indicated in bold red) and other related Type Ia, Type Ib, and Type Ic gammaproteobacterial methanotrophs were inferred using the Maximum Likelihood method based on the Dayhoff matrix-based model. Initial tree(s) for the heuristic search were obtained automatically by applying the Maximum Parsimony method. Bootstrap values (1,000 replicates) less than 40% are not shown. Evolutionary analyses were conducted in MEGA7. The Type IIb methanotroph, Methylocapsa acidiphila B2T (AJ278727) of the class Alphaproteobacteria (in the family Beijerinkiaceae) was used as outgroup. Bar, 0.10 substitutions per amino acid position.
Citation
Islam, T., Larsen, Ø., and Birkeland, N.-K. (2020) A Novel Cold-adapted Methylovulum species, with a High C16:1ω5c Content, Isolated from an Arctic Thermal Spring in Spitsbergen. Microbes Environ 35: ME20044.
https://doi.org/10.1264/jsme2.ME20044
Supplementary Material
Acknowledgements
We are grateful to Professor Lise Øvreås for laboratory support. This study was supported by the Research Council of Norway (Grant No. 157346 and 261923). Electron microscopy imaging was performed at the Molecular Imaging Center (MIC), Department of Biomedicine, University of Bergen.
References
- Adekambi T., Shinnick T.M., Raoult D., and Drancourt M. (2008) Complete rpoB gene sequencing as a suitable supplement to DNA-DNA hybridization for bacterial species and genus delineation. Int J Syst Evol Microbiol 58: 1807–1814. [DOI] [PubMed] [Google Scholar]
- Bale N.J., Rijpstra W.I.C., Sahonero-Canavesi D.X., Oshkin I.Y., Belova S.E., Dedysh S.N., and Sinninghe Damste J.S. (2019) Fatty acid and hopanoid adaption to cold in the methanotroph Methylovulum psychrotolerans. Front Microbiol 10: 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter N.J., Hirt R.P., Bodrossy L., Kovacs K.L., Embley T.M., Prosser J.I., and Murrell J.C. (2002) The ribulose-1,5-bisphosphate carboxylase/oxygenase gene cluster of Methylococcus capsulatus (Bath). Arch Microbiol 177: 279–289. [DOI] [PubMed] [Google Scholar]
- Bedard C., and Knowles R. (1989) Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev 53: 68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berestovskaya Y.Y., Vasil’eva L.V., Chestnykh O.V., and Zavarzin G.A. (2002) Methanotrophs of the psychrophilic microbial community of the Russian arctic tundra. Microbiol 71: 460–466. [PubMed] [Google Scholar]
- Bowman J.P., Mccammon S.A., and Skerratt J.H. (1997) Methylosphaera hansonii gen. nov., sp. nov, a psychrophilic, group I methanotroph from Antarctic marine-salinity, meromictic lakes. Microbiol 143: 1451–1459. [DOI] [PubMed] [Google Scholar]
- Conrad R. (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O, and NO). Microbiol Rev 60: 609–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova O.V., Kulichevskaya I.S., Rozova O.N., Detkova E.N., Bodelier P.L.E., Trotsenko Y.A., and Dedysh S.N. (2013) Methylomonas paludis sp. nov., the first acid-tolerant member of the genus Methylomonas, from an acidic wetland. Int J Syst Evol Microbiol 63: 2282–2289. [DOI] [PubMed] [Google Scholar]
- Dedysh S.N., Berestovskaya Y.Y., Vasylieva L.V., Belova S.E., Khmelenina V.N., Suzina N.E., et al. (2004) Methylocella tundrae sp. nov., a novel methanotrophic bacterium from acidic tundra peatlands. Int J Syst Evol Microbiol 54: 151–156. [DOI] [PubMed] [Google Scholar]
- Deutzmann J.S., Hoppert M., and Schink B. (2014) Characterization and phylogeny of a novel methanotroph, Methyloglobulus morosus gen. nov., spec. nov. Syst Appl Microbiol 37: 165–169. [DOI] [PubMed] [Google Scholar]
- Dunfield P.F., Yuryev A., Senin P., Smirnova A.V., Stott M.B., Hou S.B., et al. (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450: 879–882. [DOI] [PubMed] [Google Scholar]
- Etiope G., and Klusman R.W. (2002) Geologic emissions of methane to the atmosphere. Chemosphere 49: 777–789. [DOI] [PubMed] [Google Scholar]
- Fiebig J., Woodland A.B., Spangenberg J., and Oschmann W. (2007) Natural evidence for rapid abiogenic hydrothermal generation of CH4. Geochim Cosmochim Acta 71: 3028–3039. [Google Scholar]
- Frindte K., Maarastawi S.A., Lipski A., Hamacher J., and Knief C. (2017) Characterization of the first rice paddy cluster I isolate, Methyloterricola oryzae gen. nov., sp. nov. and amended description of Methylomagnum ishizawai. Int J Syst Evol Microbiol 67: 4507–4514. [DOI] [PubMed] [Google Scholar]
- Ghashghavi M., Belova S.E., Bodelier P.L.E., Dedysh S.N., Kox M.A.R., Speth D.R., et al. (2019) Methylotetracoccus oryzae strain C50C1 is a novel type Ib gammaproteobacterial methanotroph adapted to freshwater environments. mSphere 4: e00631-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R.S., and Hanson T.E. (1996) Methanotrophic bacteria. Microbiol Rev 60: 439–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama H., Fuse H., Abe M., Miyazaki M., Nakamura T., Nunoura T., et al. (2013). Methylomarinum vadi gen. nov., sp. nov., a methanotroph isolated from two distinct marine environments. Int J Syst Evol Microbiol 63: 1073–1082. [DOI] [PubMed] [Google Scholar]
- Hirayama H., Abe M., Miyazaki M., Nunoura T., Furushima Y., Yamamoto H., and Takai K. (2014) Methylomarinovum caldicuralii gen. nov., sp. nov., a moderately thermophilic methanotroph isolated from a shallow submarine hydrothermal system, and proposal of the family Methylothermaceae fam. nov. Int J Syst Evol Microbiol 64: 989–999. [DOI] [PubMed] [Google Scholar]
- Houghton K.M., Carere C.R., Stott M.B., and Mcdonald I.R. (2019) Thermophilic methanotrophs: in hot pursuit. FEMS Microbiol Ecol 95: fiz125. [DOI] [PubMed] [Google Scholar]
- Iguchi H., Yurimoto H., and Sakai Y. (2010) Soluble and particulate methane monooxygenase gene clusters of the type I methanotroph Methylovulum miyakonense HT12. FEMS Microbiol Lett 312: 71–76. [DOI] [PubMed] [Google Scholar]
- Islam T., Jensen S., Reigstad L.J., Larsen Ø., and Birkeland N.K. (2008) Methane oxidation at 55 degrees C and pH 2 by a thermoacidophilic bacterium belonging to the Verrucomicrobia phylum. Proc Natl Acad Sci U S A 105: 300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T., Larsen Ø., Torsvik V., Øvreås L., Panosyan H., Murrell J.C., et al. (2015) Novel methanotrophs of the family Methylococcaceae from different geographical regions and habitats. Microorganisms 3: 484–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T., Torsvik V., Larsen Ø., Bodrossy L., Øvreås L., and Birkeland N.K. (2016) Acid-tolerant moderately thermophilic methanotrophs of the class Gammaproteobacteria isolated from tropical topsoil with methane seeps. Front Microbiol 7: 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T., Gessesse A., Garcia-Moyano A., Murrell J.C., and Øvreås L. (2020) A novel moderately thermophilic type Ib methanotroph isolated from an alkaline thermal spring in the Ethiopian Rift Valley. Microorganisms 8: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhnaya M.G., Khmelenina V.N., Kotelnikova S., Holmquist L., Pedersen K., and Trotsenko Y.A. (1999) Methylomonas scandinavica sp. nov., a new methanotrophic psychrotrophic bacterium isolated from deep igneous rock ground water of Sweden. Syst Appl Microbiol 22: 565–572. [DOI] [PubMed] [Google Scholar]
- Kamagata Y., and Mikami E. (1991) Isolation and characterization of a novel thermophilic Methanosaeta strain. Int J Syst Bacteriol 41: 191–196. [DOI] [PubMed] [Google Scholar]
- Khalifa A., Lee C.G., Ogiso T., Ueno C., Dianou D., Demachi T., et al. (2015) Methylomagnum ishizawai gen. nov., sp. nov., a mesophilic type I methanotroph isolated from rice rhizosphere. Int J Syst Evol Microbiol 65: 3527–3534. [DOI] [PubMed] [Google Scholar]
- Kip N., Ouyang W.J., Van Winden J., Raghoebarsing A., Van Niftrik L., Pol A., et al. (2011) Detection, isolation, and characterization of acidophilic methanotrophs from Sphagnum mosses. Appl Environ Microbiol 77: 5643–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knief C. (2015) Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front Microbiol 6: 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., and Tamura K. (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen S.E., and Bottrell S. (1994) Microbiological activity in thermoglacial karst springs, South Spitsbergen. Geomicrobiol J 12: 161–173. [Google Scholar]
- Mesbah M., Premachandran U., and Whitman W.B. (1989) Precise measurement of the G+C content of deoxyribonucleic acid by high-performance liquid-chromatography. Int J Syst Bacteriol 39: 159–167. [Google Scholar]
- Murray M.G., and Thompson W.F. (1980) Rapid isolation of high molecular-weight plant DNA. Nucl Acids Res 8: 4321–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazaries L., Murrell J.C., Millard P., Baggs L., and Singh B.K. (2013) Methane, microbes and models: fundamental understanding of the soil methane cycle for future predictions. Environ Microbiol 15: 2395–2417. [DOI] [PubMed] [Google Scholar]
- Omelchenko M.V., Vasileva L.V., Zavarzin G.A., Saveleva N.D., Lysenko A.M., Mityushina L.L., et al. (1996) A novel psychrophilic methanotroph of the genus Methylobacter. Microbiology 65: 339–343. [Google Scholar]
- Op Den Camp H.J.M., Islam T., Stott M.B., Harhangi H.R., Hynes A., Schouten S., et al. (2009) Environmental, genomic and taxonomic perspectives on methanotrophic Verrucomicrobia. Environ Microbiol Rep 1: 293–306. [DOI] [PubMed] [Google Scholar]
- Orata F.D., Meier-Kolthoff J.P., Sauvageau D., and Stein L.Y. (2018) Phylogenomic analysis of the gammaproteobacterial methanotrophs (Order Methylococcales) Calls for the reclassification of members at the genus and species levels. Front Microbiol 9: 3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshkin I.Y., Belova S.E., Danilova O.V., Miroshnikov K.K., Rijpstra W.I.C., Damsté J.S.S., et al. (2016) Methylovulum psychrotolerans sp. nov., a cold-adapted methanotroph from low-temperature terrestrial environments, and emended description of the genus Methylovulum. Int J Syst Evol Microbiol 66: 2417–2423. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Chuvochina M., Waite D.W., Rinke C., Skarshewski A., Chaumeil P.A., and Hugenholtz P. (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36: 996–1004. [DOI] [PubMed] [Google Scholar]
- Prior S.D., and Dalton H. (1985) Acetylene as a suicide substrate and active-site probe for methane monooxygenase from Methylococcus-capsulatus (Bath). FEMS Microbiol Lett 29: 105–109. [Google Scholar]
- Rahalkar M., Bussmann I., and Schink B. (2007) Methylosoma difficile gen. nov., sp. nov., a novel methanotroph enriched by gradient cultivation from littoral sediment of Lake Constance. Int J Syst Evol Microbiol 57: 1073–1080. [DOI] [PubMed] [Google Scholar]
- Reeburgh W.S. (2007) Oceanic methane biogeochemistry. Chem Rev 107: 486–513. [DOI] [PubMed] [Google Scholar]
- Reigstad L.J., Jørgensen S.L., Lauritzen S.E., Schleper C., and Urich T. (2011) Sulfur-oxidizing chemolithotrophic proteobacteria dominate the microbiota in high Arctic thermal springs on Svalbard. Astrobiology 11: 665–678. [DOI] [PubMed] [Google Scholar]
- Schloss P.D., and Handelsman J. (2004) Status of the microbial census. Microbiol Mol Biol Rev 68: 686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp C.E., Smirnova A.V., Graham J.M., Stott M.B., Khadka R., Moore T.R., et al. (2014) Distribution and diversity of Verrucomicrobia methanotrophs in geothermal and acidic environments. Environ Microbiol 16: 1867–1878. [DOI] [PubMed] [Google Scholar]
- Stoecker K., Bendinger B., Schoning B., Nielsen P.H., Nielsen J.L., Baranyi C., et al. (2006) Cohn’s Crenothrix is a filamentous methane oxidizer with an unusual methane monooxygenase. Proc Natl Acad Sci U S A 103: 2363–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina P.L., Hatzenpichler R., Mcglynn S., Chadwick G., Dawson K.S., Connon S.A., and Orphan V.J. (2015) Methyloprofundus sedimenti gen. nov., sp. nov., an obligate methanotroph from ocean sediment belonging to the ‘deep sea-1’ clade of marine methanotrophs. Int J Syst Evol Microbiol 65: 251–259. [DOI] [PubMed] [Google Scholar]
- Trotsenko Y.A., and Khmelenina V.N. (2005) Aerobic methanotrophic bacteria of cold ecosystems. FEMS Microbiol Ecol 53: 15–26. [DOI] [PubMed] [Google Scholar]
- Trotsenko Y.A., and Murrell J.C. (2008) Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63: 183–229. [DOI] [PubMed] [Google Scholar]
- Van Teeseling M.C.F., Pol A., Harhangi H.R., Van Der Zwart S., Jetten M.S.M., and Op Den Camp H.J.M. (2014) Expanding the Verrucomicrobial methanotrophic world: Description of three novel species of Methylacidimicrobium gen. nov. Appl Environ Microbiol 80: 6782–6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigliotta G., Nutricati E., Carata E., Tredici S.M., De Stefano M., Pontieri P., et al. (2007) Clonothrix fusca Roze 1896, a filamentous, sheathed, methanotrophic gamma-proteobacterium. Appl Environ Microbiol 73: 3556–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobev A.V., Baani M., Doronina N.V., Brady A.L., Liesack W., Dunfield P.F., and Dedysh S.N. (2011) Methyloferula stellata gen. nov., sp. nov., an acidophilic, obligately methanotrophic bacterium that possesses only a soluble methane monooxygenase. Int J Syst Evol Microbiol 61: 2456–2463. [DOI] [PubMed] [Google Scholar]
- Wartiainen I., Hestnes A.G., Mcdonald I.R., and Svenning M.M. (2006) Methylobacter tundripaludum sp. nov., a methane-oxidizing bacterium from Arctic wetland soil on the Svalbard islands, Norway (78° N). Int J Syst Evol Microbiol 56: 109–113. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Phillips K.C., and Wilkinso J.F. (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61: 205–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.