Abstract
INTRODUCTION:
Gall bladder cancer (GBC) tends to present in advanced stages, therefore, early diagnosis of GBC is necessary. There is no ideal single tumor marker available presently for the diagnosis and prognosis of GBC. Platelet distribution width (PDW) is an early marker for activated platelets and has been used in a variety of tumors to assess prognosis. This study was designed to evaluate the utility of PDW in identifying GBC patients and its association with tumor markers, staging and resectability of GBC.
MATERIALS AND METHODS:
This cross sectional study was done on 100 patients of GBC and 100 age- and sex- matched healthy controls. PDW was evaluated and compared between GBC and healthy controls. Receiver-operating characteristics was plotted to determine optimal cut-off for identifying GBC patients and to determine sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of PDW. Correlation between serum tumor markers (carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125) and PDW were evaluated. Association of PDW with hyperbilirubinemia, staging and resectability of GBC was also studied.
RESULTS:
A significantly higher PDW with a median of 18.1 was observed in GBC as compared to healthy controls with median value of 13. PDW was found to have a very high sensitivity (90%), specificity (95%), PPV (94%) and NPV (90%) in identifying GBC at cut-off of 16 with area under the curve (AUC) of 0.97. An increase of PDW was observed with increasing stage and unresectable GBC. However, it was not statistically significant. Significant positive correlation was observed between PDW and all three serum tumor markers and good positive correlation with r = 0.61 was observed with CA 19-9.
CONCLUSION:
PDW was associated with GBC and may be considered as a cost- effective marker in adjunct to other investigations for the diagnosis of GBC.
Keywords: Gall bladder cancer, platelet distribution width, resectability, tumor markers
Introduction
Gall bladder cancer (GBC) is the most common biliary tract malignancy.[1] GBC tends to present in advanced stages with <10% of patients having resectable disease at the time of diagnosis.[2] Diagnosis of GBC is made by non-invasive imaging like contrast enhanced computer tomography of the abdomen and invasive tests such as laparoscopy and biopsy. Late diagnosis of GBC is one of the common causes of its dismal prognosis. Tumor markers available presently for the diagnosis and prognosis of GBC have been used with inconsistent results.[3] Therefore, there is an impending need for the diagnosis of GBC. Platelets play a key role in progression, induce endothelial proliferation and new vessel formation leading to angiogenesis. Platelet related proteins released facilitate proteolysis which enhances tumor growth and metastasis.[4] Platelet distribution width (PDW) is one of the platelet indices to determine the activity of platelets. It is the coefficient of variation of the average of platelet volume. Higher values of PDW indicate labile volumetric difference when compared with normal individuals. PDW has been associated with poor prognosis in many cancers including hepatocellular,[5] pancreatic,[6,7] malignant melanoma,[8] thyroid,[9,10] laryngeal[11] colorectal,[12,13,14] gastric,[15] non-small cell lung carcinoma[16] and endometrial carcinoma.[17] Studies of PDW association with GBC are scarce. Zhang et al.[18] have studied the association of PDW and mean platelet volume with GBC and found higher PDW was associated with GBC than controls. However, to the best of our knowledge, the utility of PDW as a diagnostic marker and its association with different stages and resectability and serum tumor markers in GBC has not been studied yet. In the present study we have identified the diagnostic value of PDW and its association with staging, tumor markers and resectability in GBC.
Materials and Methods
Patient selection
This cross-sectional study was conducted between January 2018 and July 2019 in the HPB unit. One hundred patients of diagnosed patients of GBC were included in this study. Inclusion criteria were age >18 years and biopsy or fine-needle aspiration cytology proven cases of GBC. Exclusion criteria were synchronous or metachronous malignancy, history of Cerebro Vascular Accident, Coronary Heart Disease, Peripheral Occlusive Vascular Disease, Chronic Liver Disease and Chronic Kidney Disease, haematological disorders and medications such as anticoagulant, statins and acetylic salicylic acid. One hundred age- and sex- matched healthy controls were also included in the study. This study was approved by the Institutional Review Board (No: AIIMS/ IEC/18/176) and all subjects provided written informed consent.
Data collection
Data included history, complete blood count, liver function and kidney function test, serum CA-125, CA 19-9, carcinoembryonic antigen (CEA) levels, contrast enhanced computerized tomography (CECT) Abdomen. All patients were staged according to eighth edition of the American Joint Committee on Cancer. Biopsy from gall bladder lesion in unresectable GBC and histopathology from radical cholecystectomy specimen of resectable GBC was done.
Sample collection
Whole blood samples were collected in ethylenediaminetetraacetic acid vials and hemoglobin, white blood cell count and platelet indices were analyzed with Beckman coulter LH-750/Sysmax XN-1000 coulter. In Sysmax XN-1000 five-part differential cell counters, the impedance principle is used for platelet count. Impedance counting discriminates particles based on their size. As platelets pass singly through an aperture between positive and negative electrodes, passing of the cell reverses the resistance and a pulse is generated which is recorded. Each pulse size is proportional to the size of platelets. Increase in PDW is an indication of platelet anisocytosis. All samples with abnormal values were also cross-checked by peripheral blood smear examination for which Leishman stain was used. Serum tumor markers- CA19-9, CEA and CA 125 were analyzed by chemiluminescence assay on Siemens Advia Centaur XP analyzer.
Statistical analysis
The data was analyzed using IBM®SPSS® software, version 23.0 (IBM Corporation, Armonk, NY, USA) Categorical variables were reported as proportion. Association between 2 categorical tests was assessed using Chi square Test. Quantitative values were reported as median and interquartile range. The Shapiro Wilk normality test showed the skewed distribution of PDW among GBC patients. Non-parametric Mann-Whitney U test was used to compare PDW among GBC cases and controls. Receiver-operating characteristics (ROC) curve analysis was performed to determine the utility of PDW in identifying GBC patients and to determine optimal cut-off value of PDW with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Non-parametric Kruskal-Wallis H test was used to compare PDW among different stages of GBC and Spearman’s correlation was performed to assess correlation between PDW and serum tumor markers. Mann-Whitney U test was used to compare PDW among resectable and unresectable GBC. P < 0.05 was considered statistically significant.
Results
A total 200 samples were included in the study, 100 GBC patients and 100 healthy control group. In cases group, there were 20 males and 80 females. In the control group, there were 22 males and 78 females. The mean age of cases was 53.4 years and mean age of controls was 53.9 years. Sociodemographic profile of patients is given in Table 1. The most common site of metastasis was found to be in the liver in 25 patients (25%), followed by involvement of the para- aortic lymph node in 24 patients (24%) and ascites 23 patients (23%) respectively. 75 (75%) of patients of carcinoma gall bladder were found unresectable. Patients with resectable disease underwent radical cholecystectomy. Unresectable patients were considered for chemotherapy. Median tumor markers values were CA 19-9: 205.1U/ml, CEA: 5.1 ng/ml and CA-125: 56.2 U/ml respectively.
Table 1.
Sociodemographic profile of patients
| Characteristic | n=100 |
|---|---|
| Age (years) | 53.4±10.4 |
| Gender | |
| Females | 78 (78%) |
| Males | 22 (22%) |
| Personal history | |
| Smoking | 35 (35%) |
| Alcohol | 14 (14%) |
| None | 41 (41%) |
| Symptoms | |
| Pain | 94 (94%) |
| Jaundice | 48 (48%) |
| Fever | 30 (30%) |
| Abdominal pain | 23 (23%) |
| Gall bladder | |
| Mass | 51 (51%) |
| Polypoidal growth | 12 (12%) |
| Wall thickening | 34 (34%) |
| Others | 3 (3%) |
| Stones | |
| Absent | 55 (55%) |
| Present | 45 (45%) |
| Liver infiltration | |
| Absent | 17 (17%) |
| Present | 83 (83%) |
| Lymph nodes | |
| Absent | 39 (39%) |
| Present | 61 (61%) |
| Metastasis | |
| Absent | 25 (25%) |
| Present | 75 (75%) |
| Bone | 1 (1%) |
| Lung | 2 (2%) |
| Omental | 6 (6%) |
| Malignant ascites | 23 (23%) |
| Liver | 25 (25%) |
| Nonregional nodes | 24 (24%) |
| Resectable | |
| No | 75 (75%) |
| Yes | 25 (25%) |
| Stage | |
| I | 1 (1%) |
| II | 10 (10%) |
| III | 14 (14%) |
| IV | 75 (75%) |
Platelet distribution width as a diagnostic marker
A significantly higher PDW was observed in GBC as compared to healthy controls PDW was found to be 18.1 (Median) and 13.25 in controls with respective 25% quartiles of 16.63 in cases and 12.10 in controls and 75% quartiles of 18.78 in cases and 14 in controls. Mann -Whitney U test showed a significant difference between cases and controls as shown in box plot [Figure 1].
Figure 1.

Box plot showing significantly higher platelet distribution width in cases of gall bladder cancer than controls
ROC analysis showed significantly high area under the curve (AUC) of 0.97 showing very good discriminatory power of PDW to identify GBC from controls [Figure 2]. At an optimal cut-off of 16 as determined by Youden index, Sensitivity, Specificity, (PPV) and (NPV) were found to be 0.90, 0.95, 0.94 and 0.90 respectively indicating PDW to be a good diagnostic marker with high sensitivity and specificity.
Figure 2.
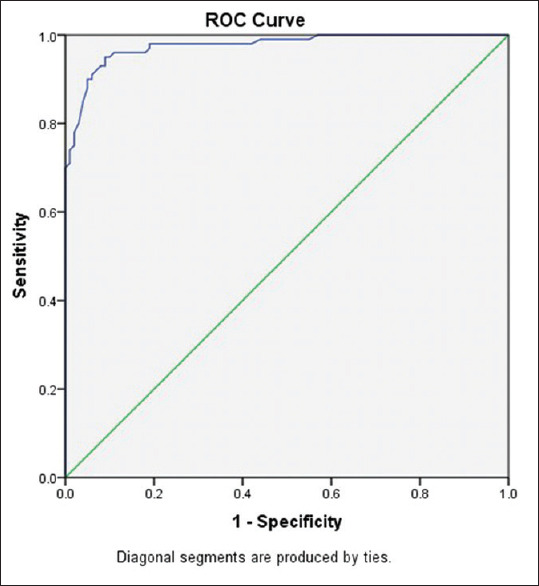
Receiver-operating characteristics curve depicting platelet distribution width in gall bladder cancer patients
Platelet distribution width correlation with tumor markers in gall bladder carcinoma
PDW correlation with tumor markers was checked using Spearman correlation and statistically significant association was seen with all tumor marker (CEA, CA 19-9 and CA 125). However, a good positive correlation with r = 0.61 was observed with CA 19-9 [Table 2].
Table 2.
Correlation of platelet distribution width with tumor markers in gall bladder cancer
| PDW | CEA | CA-19.9 | CA-125 |
|---|---|---|---|
| Spearman correlation r | 0.30 | 0.61 | 0.278 |
| P (twotailed) | 0.011* | <.0001* | 0.0223* |
| n | 74 | 86 | 71 |
*Correlation is significant at the 0.05 level (two-tailed). PDW: Platelet distribution width, CEA: Carcinoembryonic antigen
Association of platelet distribution width with staging and resectability in the carcinoma gall bladder
PDW values increased with increasing stage. Median values of PDW in stage I, II, III and IV of GBC were found to be 12.90, 14.40 15.30 and 17.3 for stage I, II, III, IV respectively However, the difference was not statistically significant (P = 0.593) [Figure 3] A higher level of PDW was observed in unresectable GBC as compared to resectable GBC. However, the difference was statistically non-significant. The median value of PDW in resectable cases of GBC was found to be 17.60, with 25% percentile of 16.48 and 75% percentile of 18.83, while the median value of unresectable cases was found to be 18.10, with 25% percentile of 16.65 and 75% percentile of 18.78 [Figure 4]. No statistically significant association was found between PDW and resectability of GBC using Mann- Whitney U Test.
Figure 3.
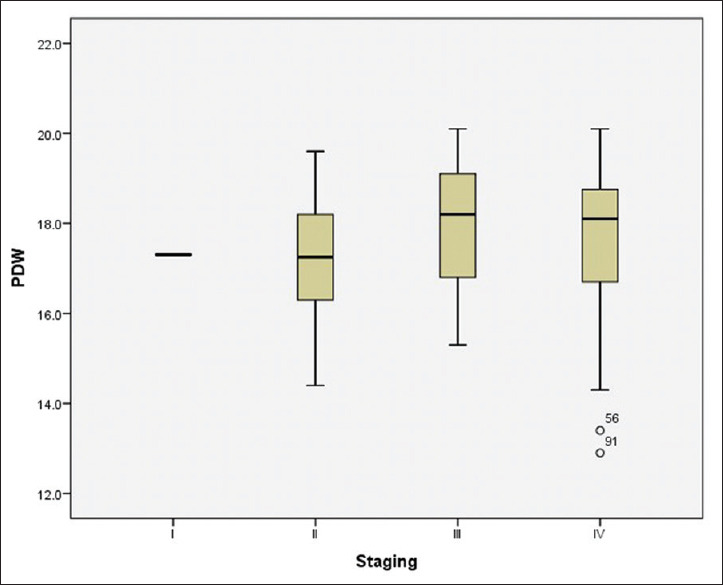
Box plot showing distribution of platelet distribution width with stages of gall bladder cancer
Figure 4.

Box plot showing distribution of platelet distribution width between resectable versus non resectable in gall bladder cancer
Discussion
Platelets plays a significant role in inflammation as well as tumor angiogenesis. Platelets activation may be assessed by various platelet indices. PDW is emerging as one of the important platelet indices in determining prognosis in a variety of cancers. It has been suggested that high PDW were found to be associated with higher recurrence risk, advanced stages of cancer and metastasis. Postulated mechanisms of raised levels of PDW in malignancy include the interplay between tumor microenvironment and activated platelets. Tumor cells secrete cytokines such as interleukin-6 (IL-6), granulocytes colony- stimulating factor (G-CSF) and macrophage CSF (M-CSF) that stimulate megakaryopoiesis. Activated platelets in turn stimulate hypercoagulable state in cancer which causes platelet to cover the tumor cells enabling them to evade the host immune system. Thereby, facilitating cancer progression and metastasis. PDW being a known marker of activated platelets may thus be associated with the prognosis of cancers.[19,20]
In the present study, PDW proved out to be a highly sensitive and specific marker with good positive and NPV at a cut-off of 16.0 and AUC of 0.97 as determined by the ROC plot. PDW showed an increasing trend with increasing stage and unresectability. However as most of the cases presenting to hospitals were in advanced stage, this could have resulted in nonsignificant difference amongst them. With regard to serum tumor markers, a positive significant correlation was observed with all the three markers. However, CA 19-9 showed the best correlation among the three markers. This is interesting as among the three studied tumor markers, CA 19-9 has shown to be the principle and most specific tumor marker in GBC.
Numerous studies have reported the association of PDW in other cancers, but studies in GBC are limited. On extensive search of indexed literature only one study could be found studying the association of PDW with GBC. The study was done by Zhang et al. It showed significant correlation between PDW and lymph node metastasis. It was concluded that elevated PDW was associated with GBC. However, no analysis was done regarding sensitivity and specificity of PDW in identifying GBC or to determine its association with staging and resectability of the disease or to correlate it with serum tumor markers in GBC.[18] PDW has shown inconsistent result in different Cancers. It has been shown to be increased while decreased in some type of cancers. A meta-analysis on breast cancer concluded increased platelet lymphocyte ratio to be associated with poor survival in breast cancer. Moreover, they concluded Asians to have poor overall survival with higher PDW in breast cancer as compared to non-Asians.[21] Another study reported PDW to be an independent marker for predicting breast cancer.[22] One of the studies in lung cancer showed significantly higher PDW value in the cancer group compared to the control group However, another study in Lung Cancer showed the association of high plateletocrit with poor survival whereas no correlation of PDW was observed with survival.[23] In the case of thyroid cancers, lower PDW has been found to be associated with poor outcomes.[24] In the case of gastrointestinal malignancies like gastric cancer, increase PDW was found to be associated with metastasis.[25] Inconsistency in PDW association with the cancers may partly be attributed to type of cancer and partly to ethnicity.
PDW however has been extensively studied in other hepatobiliary and gastrointestinal cancers. Similar to our study, PDW was found to be a diagnostic marker in Colorectal cancer in a study by Kilincalp et al.[26] However, contrary to the present study, they observed a significant association with the stage of cancer. Diagnostic importance of PDW with high AUC, sensitivity and specificity has also been shown in a previous study on hepatocellular[5] and pancreatic cancer.[6] Hence PDW can be used as an effective tumor marker in patients of GBC with reasonably good sensitivity and specificity. Added advantage of PDW being cheap, quick and easy availability. Limitation of the current study is less number of patients in early-stage due to which conclusive comment on the utility of PDW with respect to stage and resectability cannot be made. However, more studies with a larger population of GBC are required for further clarification of this issue. Furthermore, a longer follow up is required to evaluate the role of PDW to assess prognosis of GBC patients.
Conclusion
PDW levels may be used along with or as an adjunct to serum tumor markers in the workup of carcinoma of gall bladder, as a cheaper and quicker alternative for the diagnostic workup and for prognostication of the disease. However, more studies are required for the same in a larger population of GBC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC) J Surg Oncol. 2008;98:485–4. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 2.Gourgiotis S, Kocher HM, Solaini L, Yarollahi A, Tsiambas E, Salemis NS. Gallbladder cancer. Am J Surg. 2008;196:252–64. doi: 10.1016/j.amjsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085–92. doi: 10.3748/wjg.v20.i14.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J Thromb Haemost. 2011;9:237–49. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 5.Zuo, Kong W, Feng L, Zhang H, Meng X, Chen W. Elevated platelet distribution width predicts poor prognosis in hepatocellular carcinoma. Cancer Biomark. 2019;24:307–13. doi: 10.3233/CBM-182076. [DOI] [PubMed] [Google Scholar]
- 6.Chadha AS, Kocak-Uzel E, Das P, Minsky BD, Delclos ME, Mahmood U, et al. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol. 2015;54:971–8. doi: 10.3109/0284186X.2014.1000466. [DOI] [PubMed] [Google Scholar]
- 7.Ulutas KT, Sarici IS, Arpaci A. Comparison of platelet distribution width and CA19-9 in resectable pancreas cancer. Med Arch. 2018;72:210–3. doi: 10.5455/medarh.2018.72.210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Na Li, Diao Z, Huang X, Niu Y, Liu T, Liuet ZP, et al. Increased platelet distribution width predicts poor prognosis in melanoma patients. Sci Rep. 2017;7:2970. doi: 10.1038/s41598-017-03212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dincel O, Bayraktar C. Evaluation of platelet indices as a useful marker in papillary thyroid carcinoma. Bratisl Lek Listy. 2017;118:153–5. doi: 10.4149/BLL_2017_030. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Fu S, Cui MM, Niu Y, Li B, Liu ZP, et al. Platelet Distribution Width and Serum Albumin Levels for Discrimination of Thyroid Cancer From Benign Thyroid Nodules, Asian Pacific Journal of Cancer Prevention. 2017;18:1773–7. doi: 10.22034/APJCP.2017.18.7.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Liu L, Fu S, Liu YS, Wang C, Liu T, et al. Higher platelet distribution width predicts poor prognosis in laryngeal cancer. Oncotarget. 2017;8:48138–44. doi: 10.18632/oncotarget.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu X, Cao Y, Lu P, Kang Y, Lin Z, Hao T, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer. Scientific Reports 8. 2018:11814. doi: 10.1038/s41598-018-29293-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagoz B, Sucullu I, Sayan O, Bilgi O, Tuncel T, Filiz AI, et al. Platelet indices in patients with colorectal cancer. Eur J Med. 2010;5:365–8. [Google Scholar]
- 14.Li JY, Li Y, Jiang Z, Wang RT, Wang XS. Elevated mean platelet volume is associated with presence of colon cancer. Asian Pac J Cancer Prev. 2014;15:10501–4. doi: 10.7314/apjcp.2014.15.23.10501. [DOI] [PubMed] [Google Scholar]
- 15.Cheng S, Han F, Wang Y, Xu Y, Qu T, JU Y, et al. The red distribution width and the platelet distribution width as prognostic predictors in gastric cancer. BMC Gastroenterol. 2017;17:163. doi: 10.1186/s12876-017-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui MM, Li N, Liu X. Platelet distribution width correlates with prognosis of non-small cell lung cancer. Sci Rep. 2017;7:3456. doi: 10.1038/s41598-017-03772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac J Cancer Prev. 2014;15:10231–6. doi: 10.7314/apjcp.2014.15.23.10231. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Neu Y, Wang X, Liu Z, Liu T, Wang RT. Mean Platelet Volume and Platelet Distribution Width Are Associated with Gallbladder Cancer, Asian Pacific Journal of Cancer Prevention. 2018;19:351–35. doi: 10.22034/APJCP.2018.19.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: A link between thrombosis and inflammation. Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 20.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin Thromb Hemost. 2014;40:296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Y, Si W, Sun Q, Qin B, Zhao W, Yang J. Platelet-lymphocyte ratio acts as an indicator of poor prognosis in patients with breast cancer. Oncotarget. 2017;8:1023–30. doi: 10.18632/oncotarget.13714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okuturlar Y, Gunaldi M, Tiken EE, Oztosun B, Inan Y O, Ercan T, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev. 2015;16:2409–12. doi: 10.7314/apjcp.2015.16.6.2409. [DOI] [PubMed] [Google Scholar]
- 23.Oncel M, Kiyici A, Oncel M, Sunam GS, Sahin E, Adam B. Evaluation of Platelet Indices in Lung Cancer Patients. Asian Pac J Cancer Prev. 2015;16:7599–602. doi: 10.7314/apjcp.2015.16.17.7599. [DOI] [PubMed] [Google Scholar]
- 24.Yaylaci S, Tosun O, Sahin O, Genc AB, Aydin E, Demiral G, et al. Lack of variation in inflammatory hematological parameters between benign nodular goiter and papillary thyroid Cancer. Asian Pac J Cancer Prev. 2016;17:2321–3. doi: 10.7314/apjcp.2016.17.4.2321. [DOI] [PubMed] [Google Scholar]
- 25.Gunaldi M, Erdem D, Goksu S, Gunduz S, Okuturlar Y, Tiken E, et al. Platelet Distribution Width as a Predictor of Metastasis in Gastric Cancer Patients. J Gastrointest Cancer. 2016;48:341–6. doi: 10.1007/s12029-016-9886-5. [DOI] [PubMed] [Google Scholar]
- 26.Kilincalp S, Coban S, Akinci H, Hamamci M, Karaahmet F, Coskun Y, et al. Neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and mean platelet volume as potential biomarkers for early detection and monitoring of colorectal adenocarcinoma. European journal of cancer prevention. 2015;24:328–333. doi: 10.1097/CEJ.0000000000000092. [DOI] [PubMed] [Google Scholar]


