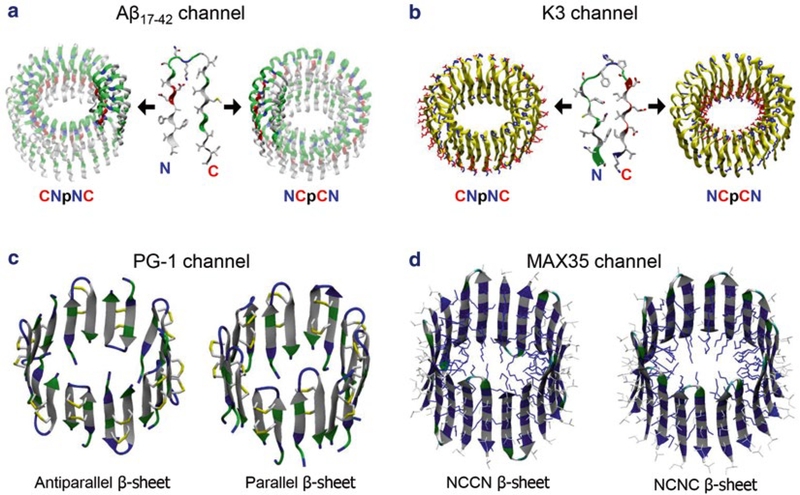
Fig. 2.
Computational models of β-sheet channel with the U-shaped and β-hairpin motifs. (a) Building annular channel structures in the membrane using (a) the NMR-based Aβ17–42 and(b) the ssNMR K320–41 peptides. In the double-layered β-sheet channels, two different directions of peptide addition along the curvature yield the CNpNC (left) and NCpCN (right) channels (here, C: C-terminal, N: N-terminal, p: pore). Unlike the U-shaped peptides, β-hairpins generate a single layered annular β-sheet for (c) protegrin-1 (PG-1) and (d) MAX 35 channels. The PG-1 channels contain the antiparallel (turn-next-to-tail, left) and parallel (turn-next-to-turn, right) β-sheet arrangements in an NCCN packing mode. In the MAX channels, the β-hairpin arrangements give rise to two potential β-sheet motifs; turn-next-to-tail β-hairpins in NCCN packing mode (left) and turn-next-to-turn β-hairpins in NCNC packing mode (right). In both cases, the MAX β-hairpins form antiparallel β-sheets, positioning the positively charged Lys side chains into the central pore