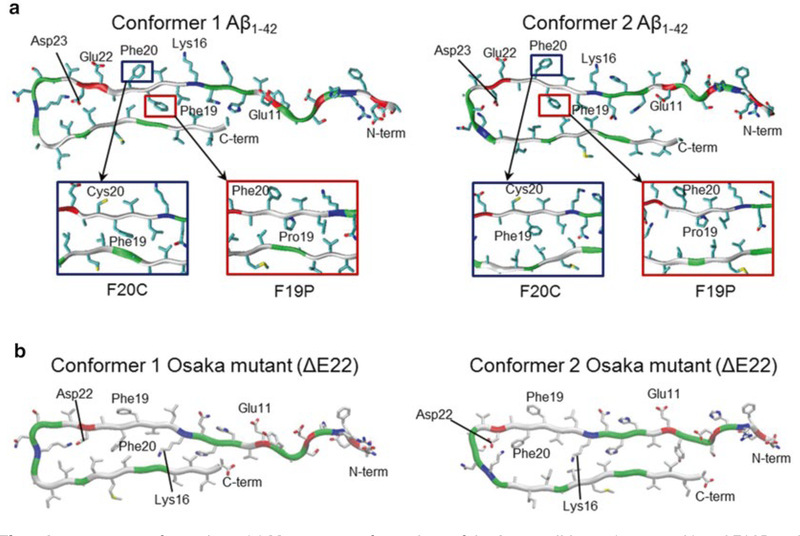
Fig. 5.
Aβ mutants conformations. (a) Monomer conformations of the Aβ1–42 wild type (top panels) and F19P and F20C mutants (highlighted in rectangular insets) with two different conformers, conformer 1 (left) with turn at Ser26-Ile31 and conformer 2 (right) with turn at Asp23-Gly29. (b) Monomer conformations of the Osaka mutant (ΔE22) with two different conformers, conformer 1 (left) and conformer 2 (right). Several important residues in the pore-lining strand are marked. In the peptide ribbon, hydrophobic, polar/Gly, positively charged, and negatively charged residues are colored white, green, blue, and red, respectively