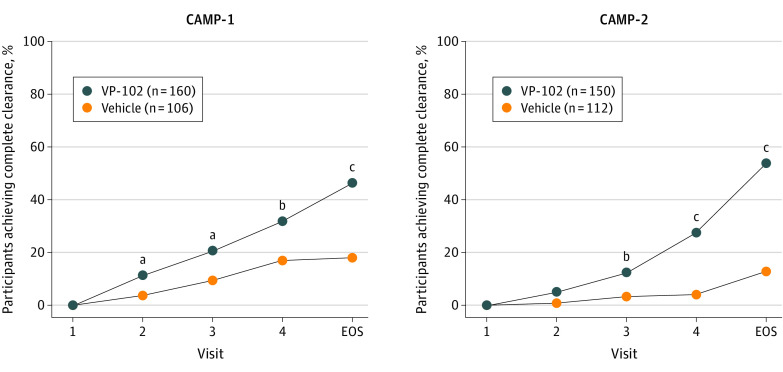
Figure 2. Percentage of Participants With Complete Clearance of Baseline and New Molluscum Contagiosum Lesions After Treatments With VP-102 or Vehicle (Statistical Comparisons at Day 21, 42, 63, or 84).
Complete clearance of all treatable lesions (baseline and new) at individual time points after treatment, treated as binary end points and tested with a Pearson χ2 test. All statistical tests were 2-sided with a significance level of α = .05. P values were completed for the intent-to-treat population at individual time points, where independent assessments were performed at days 21, 42, 63, and 84, and not meant to imply statistical significance between those time points. Individual lesions were not tracked, and total lesion counts included any treatable (baseline and new) lesions that remained on the day/visit after baseline. If participant lesions cleared before the end of study (EOS), they were required to stay clear until the EOS visit to be counted as completely clear. At the EOS visit, 46.3% of participants in the VP-102 group and 17.9% in the vehicle group were completely clear of all lesions for Cantharidin Application in Molluscum Patients (CAMP)-1 (P < .001). In CAMP-2, 54.0% of VP-102 participants vs 13.4% of the vehicle participants were completely clear at the EOS visit (P < .001). While the methods for the trials were identical, clinical sites and participants were not the same between trials, and thus there is natural variability in results for each group from one trial to the other.
aP < .05.
bP < .01.
cP < .001