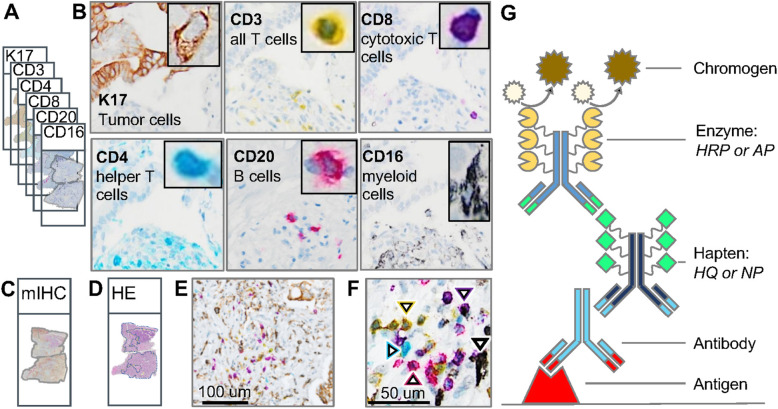
Fig. 1.
IHC markers in the PDAC mIHC panel to study tumor immune interactions. a Traditional IHC to stain six adjacent serial tissue sections with one biomarker per tissue section. Each biomarker is designated by a specific color. b Representative images from one marker IHC in serial tissue sections showing expression in the same region of interest (except for examples of sparsely distributed B-cells from a different area in the corresponding histologic section). Each inset shows the cellular expression of the corresponding marker at higher magnification. C. Multiplex IHC (mIHC) in adjacent serial section. d Hematoxylin and eosin (H&E) in adjacent serial sections with delineation of the tumor region by a pathologist. e mIHC with six IHC markers on a single tissue section with PDAC. f At the highest magnification, each of the five immune cell classes are labeled with a white arrowhead outlined in the color corresponding to chromophore color. T-cell subtypes include CD3+ (yellow), CD4+ (teal), CD8+ (purple); B-cells denoted by CD20+ (red); and myeloid cells identified by CD16+ (silver-black). g Indirect mIHC using hapten-conjugated secondary antibodies. Primary antibody binds to target antigen; secondary anti-mouse or anti-rabbit IgG antibody conjugated to synthetic haptens (HQ or NP) to bind primary antibody; and anti-hapten tertiary antibody conjugated to multiple enzyme molecules (e.g. horseradish peroxidase (HRP) or alkaline phosphatase (AP)) to bind secondary antibody. Chromogen substrate reacts with enzymes to generate insoluble unique color signal at the site of the targeted antigen