Abstract
OBJECTIVE.
The purpose of this study was to perform a systematic review and meta-analysis regarding CT features of non–small cell lung cancer (NSCLC) with anaplastic lymphoma kinase (ALK) rearrangement.
MATERIALS AND METHODS.
The PubMed and Embase databases were searched up to February 20, 2019. Studies that evaluated CT features of NSCLC with and without ALK rearrangement was included. Methodologic quality was assessed using Quality Assessment of Diagnostic Accuracy Studies–2. The association between CT features and ALK rearrangement was pooled in the form of the odds ratio (OR) or the mean difference (MD) using the random-effects model. Heterogeneity was examined using the inconsistency index (I2). Publication bias was examined using funnel plots and Egger tests.
RESULTS.
Sixteen studies were included, consisting of 3113 patients with NSCLC. The overall prevalence of patients with ALK rearrangement was 17% (528/3113). Compared with NSCLC without ALK rearrangement, on CT images those with ALK rearrangement were more frequently solid (OR = 2.86), central in location (OR = 2.72), and 3 cm or smaller (OR = 0.57); had lower contrast-enhanced CT attenuation (MD = −4.79 HU); more frequently had N2 or N3 disease (OR = 5.63), lymphangitic carcinomatosis (OR = 3.46), pleural effusion (OR = 1.91), or pleural metastasis (OR = 1.81); and less frequently had lung metastasis (OR = 0.66). Heterogeneity varied among CT features (I2 = 0–80%). No significant publication bias was seen (p = 0.15).
CONCLUSION.
NSCLC with ALK rearrangement had several distinctive CT features compared with that without ALK rearrangement. These CT biomarkers may help identify patients likely to have ALK rearrangement.
Keywords: anaplastic lymphoma kinase, CT, meta-analysis, non–small cell lung cancer, systematic review
Lung cancer is the leading cause of cancer-related deaths in the United States [1]. The discovery of several genetic alterations related with non–small cell lung cancer (NSCLC) has catalyzed development of new drugs targeting these signaling pathways. In 2007, a novel genetic alteration, anaplastic lymphoma kinase (ALK) rearrangement, was identified [2]. Although the frequency of patients with ALK rearrangement is relatively low, with reported values between 2% and 7% in patients with NSCLC, response to treatment with selective inhibitors of ALK tyrosine kinase such as crizotinib, alectinib, brigatinib, ceritinib, and lorlatinib has been shown, emphasizing the importance of recognizing these patients [3–7].
The most recent National Comprehensive Cancer Network guidelines recommend testing for genetic alterations in patients with advanced NSCLC before initial treatment, but a recent survey revealed that only 82% of physicians perform such testing [8, 9]. When tissue samples are available, specimen yield may not be sufficient to perform molecular testing if samples are obtained with minimally invasive techniques. In addition, because of the intra- and intertumoral heterogeneity of lung cancer, genetic mutation status may be inaccurate or underestimated when based on a single biopsy sample from either primary tumor or metastases [10]. Furthermore, although molecular retesting is recommended when disease progression is suspected to look for evidence of tumor genomic evolution, multiple or repeated biopsies may not be feasible in clinical practice because of logistical and financial hurdles [11].
CT is an established imaging modality routinely used for initial diagnostic staging and monitoring treatment response. Evidence is emerging that imaging features correlate with the genomic landscape of tumors, namely, radiogenomics [12]. If CT correlates of genetic aberrations such as ALK rearrangement can be found, radiologists may be able to suggest molecular testing in certain clinical situations. Although a few studies have assessed imaging characteristics of NSCLC with ALK rearrangements, definitive conclusions could not be drawn for several reasons: small number of patients; limited number of evaluated CT findings that varied among the studies; and on occasion, conflicting results between studies [13–17]. Therefore, the purpose of this study was to systematically review the literature and perform a meta-analysis regarding the CT findings of NSCLC with ALK rearrangement.
Materials and Methods
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [18]. We formulated a research question that was based on modification of the patient, index test, comparator, outcome, and study design criteria as follows: What are the CT features associated with NSCLC with ALK rearrangement compared with that without ALK rearrangement in original research articles?
Literature Search
We conducted a systematic search in the PubMed and Embase databases up to December 23, 2018, and continued updating the literature search until February 20, 2019. Keywords and their synonyms or relevant terms were included in the following search query as the following: (“computed tomography” OR CT OR HRCT OR imaging OR clinicoradiologic* OR radiologic*) AND (feature OR finding OR character* OR biomarker) AND (“lung cancer” OR “lung carcinoma” OR “lung adenocarcinoma”) AND (“anaplastic lymphoma kinase” OR ALK). The bibliographies of included studies were screened to find other eligible studies. We did not limit the search to articles written in any particular language.
Inclusion Criteria
Qualified studies were included if they satisfied the following patient, index test, comparator, outcome, and study criteria: patients diagnosed with NSCLC; ALK status was determined by fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), polymerase chain reaction (PCR), or some combination of those techniques; CT was used for characterization of primary tumor and tumor burden; the study evaluated association between ALK rearrangement and CT features or provided the corresponding raw data for constructing a 2 × 2 contingency table for categoric variables or mean and SD for continuous variables; and the publication was an original research article.
Exclusion Criteria
Studies were excluded if the study population included fewer than 10 patients; the publication was not an original research article; CT was used for evaluation of NSCLC but focused on topics other than association with ALK rearrangement status; imaging modalities other than CT were used; patient populations overlapped between different studies; or that were insufficient data for reconstruction of 2 × 2 tables or means and SDs (i.e., studies that only included patients with ALK rearrangement). If multiple publications with considerable overlapping study populations between different studies were identified, we only included the study providing a greater number of relevant data categories with regard to association between ALK rearrangement status and CT features.
Two reviewers independently conducted the literature search and study selection. If the reviewers disagreed, consensus was reached after discussion with a third reviewer.
Data Extraction and Quality Assessment
The following data regarding patient, study, and CT characteristics and imaging features were extracted using a standardized form for patient, study, and CT characteristics as well as CT features. Patient characteristics included duration of patient recruitment, number and age of patients, characteristics of patients without ALK mutation (i.e., mutation status of other genetic alterations such as EGFR or K-ras), histologic type of NSCLC, tumor stage, and detection and sampling method for ALK rearrangement status. Study characteristics consisted of origin of study (authors, institution, and country), publication year, study design (prospective vs retrospective, whether enrollment was consecutive, and multicenter or single center), and number and experience of CT readers. CT characteristics were detector number, scanner model and manufacturer, slice thickness, interval thickness, CT parameters, and use of contrast enhancement.
We assessed the methodologic quality of the included studies using the Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS-2) tool [19]. Both data extraction and quality assessment were performed independently by two reviewers with disagreement resolved after discussion with a third reviewer.
Data Synthesis and Analysis
Data from the included studies were reconstructed in 2 × 2 contingency tables showing the presence or absence of CT features in patients with and without ALK mutation for categoric variables or as means and SDs for continuous variables. If results by multiple independent readers were available, those from the more experienced reader were extracted for this meta-analysis. The association between the imaging features and ALK rearrangement of NSCLC was assessed pooled in the form of an odds ratio (OR) or the mean difference (MD) with 95% CIs using the random-effects model. Heterogeneity was examined by the inconsistency index (I2) [20]. Studies with I2 greater than 50% were considered to show significant heterogeneity [21]. Publication bias was examined for CT features that included more than 10 studies using funnel plots and Egger tests [22]. Deviation from the funnel-shaped distribution of eligible studies was considered to indicate publication bias.
The meta and metafor packages of R software (version 3.5.1, R Foundation for Statistical Computing) were used for statistical analyses with p < 0.05 indicating statistical significance.
Results
Literature Search
The systematic literature search initially identified 432 articles. After we removed 69 duplicates, screening of the 363 titles and abstracts yielded 29 potentially eligible studies. Full-text reviews were performed, and 14 studies were excluded because they had insufficient data to reconstruct ORs or MDs (n = 8), had overlapping patient populations (n = 3), or used PET/CT for assessment of NSCLC with ALK rearrangement and did not clearly explain the role of CT for the tumor assessment (n = 3). Additionally, extended search of bibliographies of included studies yielded one study eligible for our meta-analysis [15]. No additional articles were identified by the updated search on February 20, 2019, and ultimately 16 original research articles were included, consisting of 3113 patients with NSCLC, 528 of whom had ALK rearrangement [13–17, 23–33]. The detailed study selection process is shown in Figure 1.
Fig. 1—
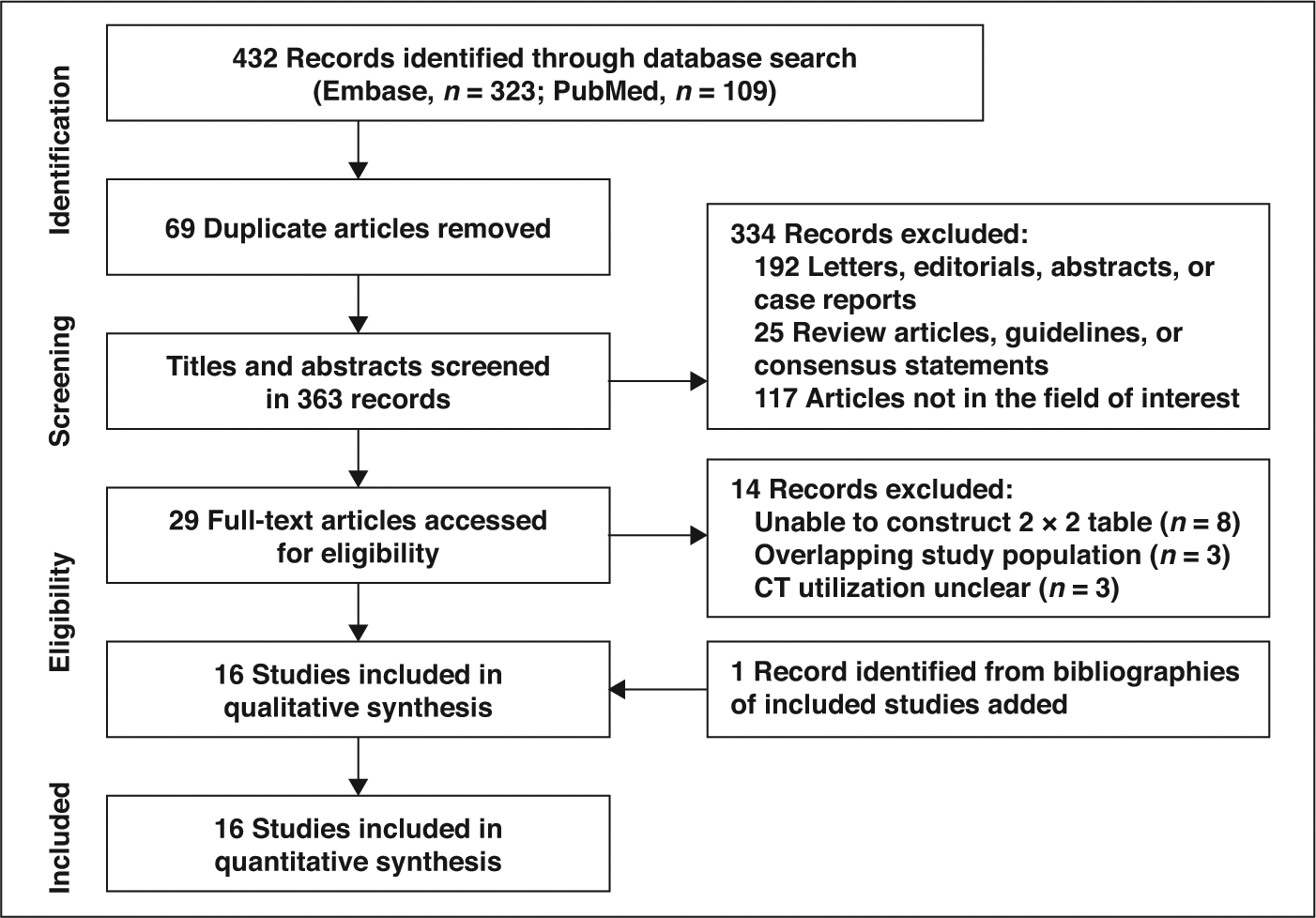
Flow diagram illustrates study selection process for meta-analysis.
Categorization and Definition of CT Features
We found 182 overlapping descriptions in 16 studies to describe various CT features. Among the overlapping descriptions, 11 descriptions that were broad in meaning, such as “intrathoracic disease,” were excluded, and 21 descriptions that were investigated in fewer than three studies were also excluded. For cases in which multiple similar descriptions were used in different studies to describe the same imaging finding, they were subsumed under a single CT feature for analysis. For example, descriptions such as “lymphangitic carcinomatosis” or “lymphangitic metastasis” were subsumed into a single description: “lymphangitic metastasis.” Finally, descriptors were subsumed under 18 CT features that included 16 categoric variables and two continuous variables that were computed in the meta-analysis. They were density (solid vs subsolid), calcification, necrosis, air bronchogram, bubblelike lucency or cavitation, lobulated margin, spiculated margin, location, size (mass [> 3 cm] vs nodule [≤ 3 cm]), pleural retraction, size (in centimeters, continuous variable), attenuation on contrast-enhanced CT (in Hounsfield units, continuous variable), lymphadenopathy, lymphangitic carcinomatosis, lung metastasis, bone metastasis, pleural effusion, and pleural metastasis.
Characteristics of Included Studies
Patient characteristics are described in Table 1. The overall prevalence of patients with ALK rearrangement was 17% (528/3113). The number of patients with ALK rearrangement on a per-study basis ranged from 10 to 68, and the number without ALK rearrangement on a per-study basis ranged from 20 to 313. The patients had a mean age of 51.0–65.4 years. Twelve studies used patients without ALK rearrangement as a control group [13, 15, 16, 23, 24, 26–30, 32, 33], and four used patients with EGFR mutation as a control group [14, 17, 25, 31]. Twelve studies included only patients with adenocarcinoma [13–17, 23, 25–27, 31–33]; four included patients with adenocarcinoma and other subtypes of NSCLC [24, 28–30]. Fifteen studies provided information regarding tumor stage. Regarding the method used to establish the presence of ALK rearrangement, nine studies used FISH [13, 14, 16, 24, 25, 27–29, 32], three used IHC [15, 17, 31], one used both IHC and FISH [26], and three used PCR, IHC, and FISH [23, 30, 33]. Tissue sampling was done using biopsy in five studies [25, 26, 30–32], surgery in five [15, 17, 23, 28, 33], and either biopsy or surgery in two [13, 27]. Four studies did not report how they procured the tissue [14, 16, 24, 29].
Table 1:
Patient Characteristics
| Study (First Author, Reference) | No. of Patients | Mean Age (Range) (y) | Comparison | Histologic Type | Tumor Stage | Method for ALK Rearrangement Assessment | Sampling Method | |
|---|---|---|---|---|---|---|---|---|
| With ALK Rearrangement | Without ALK Rearrangement | |||||||
| Choi [13]a | 18 | 313 | 62.2 (14–85) | ALK - | AC | I–IV | FISH | Biopsy, surgery |
| Choi [25] | 68 | 130 | 56.6 (27–86) | EGFR | AC | IV | FISH | Biopsy |
| Fukui [23]b | 28 | 140 | 63.0 (22–89) | ALK - | AC | I–IV | PCR, IHC, FISH | Surgery |
| Halpenny [14] | 25 | 85 | 59.8 (33–90) | EGFR | AC | I–IV | FISH | NR |
| Jeong [26] | 41 | 180 | 60.1 (NR) | ALK - | AC | Advanced | IHC, FISH | Biopsy |
| Kim [28] | 25 | 173 | 61.6 (27–91) | ALK - | AC, SCC, adenosquamous, pleomorphic, large cell carcinoma, carcinosarcoma, NOS | I–IV | FISH | Surgery |
| Miao [31] | 33 | 112 | 57.4 (27–78) | EGFR | AC | IIIB–IV | IHC | Biopsy |
| Mueller-Lisse [29] | 10 | 29 | 57.0 (NR) | ALK - | AC, NOS | IIB–IV | FISH | NR |
| Nakada [15] | 27 | 209 | NR | ALK - | AC | I-IV | IHC | Surgery |
| Park[30]b,c | 47 | 161 | 59.4 (29–89) | ALK - | AC, adenosquamous, SCC, large cell, large cell neuroendocrine, sarcomatoid carcinoma, NOS | IIIB–IV | PCR, IHC, FISH | Biopsy |
| Rizzo [16] | 31 | 239 | 65.2 (NR) | ALK - | AC | NR | FISH | NR |
| Seto [33]c | 19 | 305 | 65.4 (29–87) | ALK - | AC | 0–IIIB | PCR, IHC, FISH | Surgery |
| Wang [17] | 41 | 66 | 58.1 (NR) | EGFR | AC | I–IV | IHC | Surgery |
| Yamamoto [24]a | 47 | 125 | 64.8 (30–90) | ALK - | AC, SCC, unknown | ≤ IIIA, > IIIA, unknown | FISH | NR |
| Zhang [32] | 20 | 20 | 51.0 (24–82) | ALK - | AC | IV | FISH | Biopsy |
| Zhou [27] | 48 | 298 | 58.9 (23–83) | ALK - | AC | I–IV | FISH | Biopsy, surgery |
Note—ALK = anaplastic lymphoma kinase, ALK − = without ALK rearrangement, AC = adenocarcinoma, FISH = fluorescence in situ hybridization, EGFR = epidermal growth factor receptor gene, PCR = polymerase chain reaction, IHC = immunohistochemistry, NR = not reported, SCC = squamous cell carcinoma, NOS = not otherwise specified.
Overlap present but investigated different CT features.
Overlap present but not to a considerable degree (< 14% [29/208]).
Overlap present but not to a considerable degree (< 1% [2/324]).
Study characteristics are shown in Table 2. All 16 studies were retrospectively performed, and only one was performed at multiple centers [24]. Patient recruitment was consecutive in 12 studies [13, 16, 17, 24–31, 33] and four were case-control studies [14, 15, 23, 32]. CT acquisition parameters and CT scanner characteristics are described in Table 3.
TABLE 2:
Study Characteristics
| Study (First Author, Reference) | Year of Publication | Country | Institution | Duration of Patient Recruitment | Consecutive Enrollment | Study Design | Multicenter Study | No. of CT Readers | CT Reader Experience (y) |
|---|---|---|---|---|---|---|---|---|---|
| Choi [13] | 2013 | Korea | Seoul National University Hospital | 9/2009–9/2011 | Yes | R | No | NR | Experienced (NS) |
| Choi [25] | 2015 | Korea | Asan Medical Center | 11/2004–12/2013 | Yes | R | No | 2 | 5,18 |
| Fukui [23] | 2012 | Japan | Aichi Cancer Center | 2001–2010 | No | R | No | 2 | NR |
| Halpenny [14] | 2014 | United States | Memorial Sloan-Kettering Cancer Center | 11/2005–6/2012 | No | R | No | 2 | 5,6 |
| Jeong [26] | 2015 | Korea | Samsung Medical Center | 3/2010–2/2011 | Yes | R | No | 2 | 3,10 |
| Kim [28] | 2016 | Korea | Seoul National University Bundang Hospital | 5/2003–7/2010 | Yes | R | No | 2 | NR |
| Miao [31] | 2017 | China | Jinling Hospital | 1/2013–12/2015 | Yes | R | No | 2 | >5 |
| Mueller-Lisse [29] | 2017 | Germany | Ludwig-Maximilians-Universitat Hospital, University of Munich | 12/2010–2/2012 | Yes | R | No | 2 | 4, >10 |
| Nakada [15] | 2015 | Japan | Cancer Institute Hospital | 10/2004–12/2010 | No | R | No | Multiple (NS) | NR |
| Park [30] | 2016 | Japan | Aichi Cancer Center | 7/2006–3/2014 | Yes | R | No | 3 | NR |
| Rizzo [16] | 2016 | Italy | European Institute of Oncology | 5/2006–2/2014 | Yes | R | No | 2 | 3,11 |
| Seto [33] | 2018 | Japan | Aichi Cancer Center | 1/2012–12/2015 | Yes | R | No | NR | NR |
| Wang [17] | 2016 | China | Tianjiin Medical University Cancer Institute and Hospital | 1/2014–7/2015 | Yes | R | No | 2 | 6,9 |
| Yamamoto [24] | 2014 | Korea, United States | Seoul National University Hospital, Massachusetts General Hospital, Scottsdale Healthcare Medical Center | 3/2009–2/2013 | Yes | R | Yes | 2 | >15 |
| Zhang [32] | 2017 | China | National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College | 2011–2014 | No | R | No | 2 | NR |
| Zhou [27] | 2015 | China | First Affiliated Hospital, Zhejiang University | 3/2008–10/2013 | Yes | R | No | 2 | 15,30 |
Note—R = retrospective, NR = not reported, NS = not specified.
TABLE 3:
CT Acquisition Parameters and Scanner Characteristics
| Study (First Author, Reference) | No. of Detector Rows | Scanners Used | Slice Thickness (mm) | Interval Thickness (mm) | Tube Voltage (kVp) | Tube Current (mA) | Contrast Enhancement |
|---|---|---|---|---|---|---|---|
| Choi [13] | NR | NR | NR | NR | NR | NR | NR |
| Choi [25] | 16–64 | LightSpeed VCTa, Somatom Sensation 16b | 1.25 | 5 | 120 | 100–400 | Yes |
| Fukui [23] | NR | NR | NR | NR | NR | NR | NR |
| Halpenny [14] | Multidetector | NR | 1.25–5 | NR | NR | NR | Yes |
| Jeong [26] | 64 | LightSpeed VCTa | 2.5 | NR | 120 | 125 | Yes |
| Kim [28] | NR | NR | NR | NR | 120 | 100–150 | Yes |
| Miao [31] | 64 | Somatom Sensation 64b | 2 | 1 | 120 | 150–200 | Yes |
| Mueller-Lisse [29] | NR | NR | 3 | NR | NR | NR | NR |
| Nakada [15] | NR | NR | 1.25 | NR | NR | NR | NR |
| Park [30] | NR | NR | NR | NR | NR | NR | NR |
| Rizzo [16] | 16–64 | LightSpeeda, MSTC Optima 660a | 2.5 | NR | 120 | 80–440 | Yes |
| Seto [33] | NR | NR | 1–2 | NR | NR | NR | NR |
| Wang [17] | 16–64 | Discovery CT750 HDa, LightSpeed 16a, Somatom Sensation 64b | 1.25–1.5 | 1.25–1.5 | 120 | 150–200 | Yes |
| Yamamoto [24] | 16–64 | LightSpeed Ultraa, Sensation 16b, Brilliance 64c, MX8000c | 1–1.25 | 1–1.25 | 120 | 100–400 | Yes |
| Zhang [32] | 64 | LightSpeed 64 VCTa, Aquilion 64d | 1–1.25 | 0.8–1 | 120 | 380–450 | Yes |
| Zhou [27] | 64–256 | LightSpeed VCTa, Brilliance iCTc | 1–5 | 1–5 | 120 | 120–380 | Yes |
Note—NR = not reported.
Manufactured by GE Healthcare.
Manufactured by Siemens Healthineers.
Manufactured by Philips Healthcare.
Manufactured by Toshiba Medical Systems.
Quality Assessment
In general, the studies were considered to be of good quality, with most satisfying more than five of the seven domains (Fig. 2). Regarding the patient selection domain, four studies [14, 15, 23, 32] were considered to have high risk of bias because of their case-control study design. Four studies [14, 25, 31, 33] that analyzed patients with EGFR mutation specifically for comparison showed high risk of bias for applicability. Regarding the index test domain, risk of bias was unclear in seven studies [13, 15, 23, 26, 28, 30, 32] because patient information blinding during CT interpretation was not explicitly described. Regarding the flow and timing domain, two studies [13, 14] had unclear risk of bias because the interval between CT and histopathology was unclear.
Fig. 2—
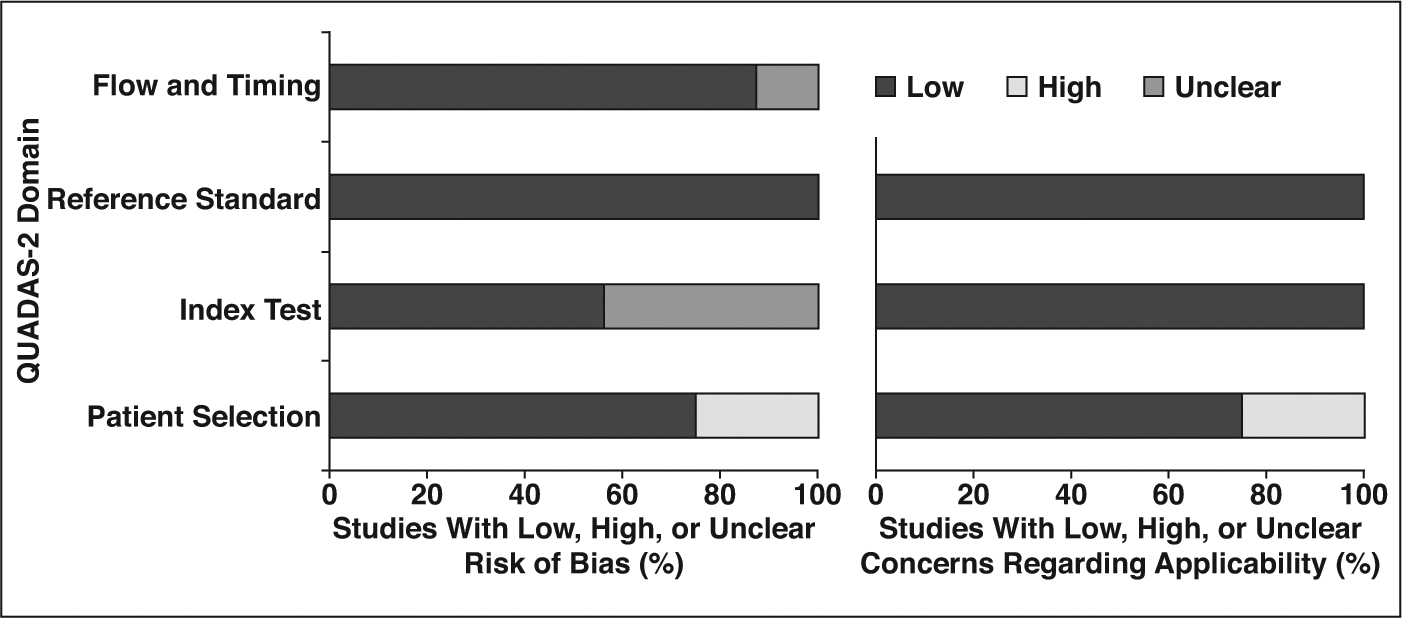
Graphs show risk of bias (left) and concerns regarding applicability (right) of 16 included studies using Quality Assessment of Diagnostic Accuracy Studies–2 (QUADAS-2).
CT Characteristics
Features of primary tumor and anaplastic lymphoma kinase rearrangement—
Four features showed significant association with ALK rearrangement (Fig. 3). NSCLC with ALK rearrangement was more frequently solid than subsolid in density compared with those without ALK rearrangement (OR = 2.86 [95% CI, 1.52–5.39], p < 0.01), was more commonly central than peripheral (OR = 2.72 [95% CI, 1.63–4.53], p < 0.01), less frequently manifested as a mass larger than 3 cm (OR = 0.57 [95% CI, 0.33–0.97], p = 0.04), and showed lower attenuation on contrast-enhanced CT (MD = −4.79 HU [95% CI, −9.10 to 0.47 HU], p = 0.03).
Fig. 3—
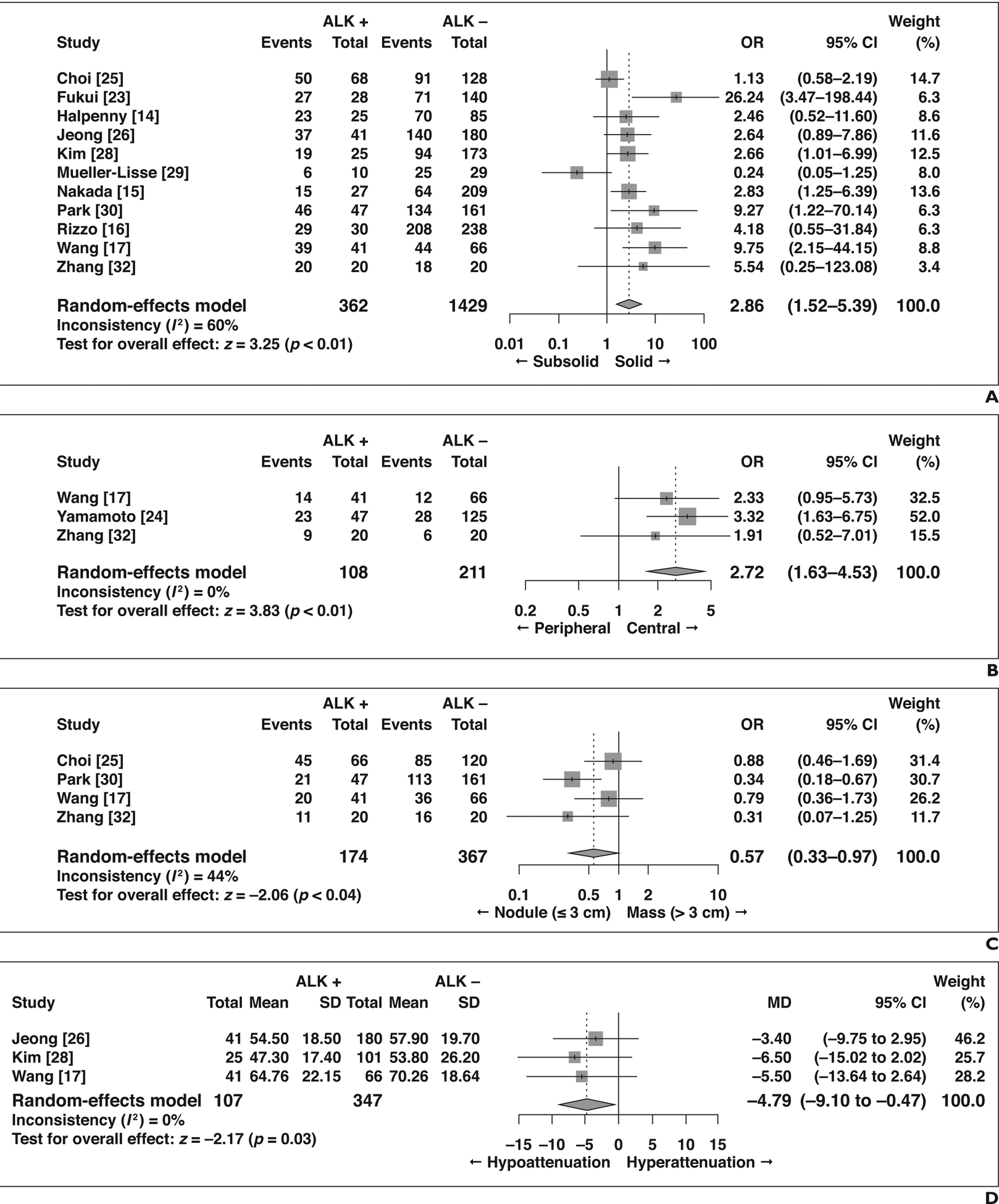
Forest plots of studies on association between CT features of primary tumor and anaplastic lymphoma kinase (ALK) rearrangement with statistical significance. Boxes indicate means, horizontal lines represent 95% CI, diamonds represent pooled indexes, and vertical dashed lines indicate pooled means. ALK + = with ALK rearrangement, ALK − = without ALK rearrangement.
A, Forest plot of odds ratio (OR) in studies assessing lesion density.
B, Forest plot of OR in studies assessing lesion location.
C, Forest plot of OR in studies assessing lesion size.
D, Forest plot of mean difference (MD) in studies assessing attenuation on contrast-enhanced CT.
The remaining eight features did not show significant association with ALK rearrangement status (Fig. 4): air bronchogram (OR = 0.79 [95% CI, 0.58–1.06], p = 0.12), spiculated margins (OR = 0.70 [95% CI, 0.39–1.26], p = 0.24), lobulated margins (OR = 1.42 [95% CI, 0.65–3.09], p = 0.37), pleural retraction (OR = 0.54 [95% CI, 0.26–1.13], p = 0.10), calcification (OR = 1.24 [95% CI, 0.60–2.59], p = 0.56), necrosis (OR = 1.15 [95% CI, 0.68–1.93], p = 0.60), bubble lucency or cavitation (OR = 0.86 [95% CI, 0.30–2.48], p = 0.79), and size measured as a continuous variable (MD = −0.95 [95% CI, −3.25 to 1.36], p = 0.42).
Fig. 4—
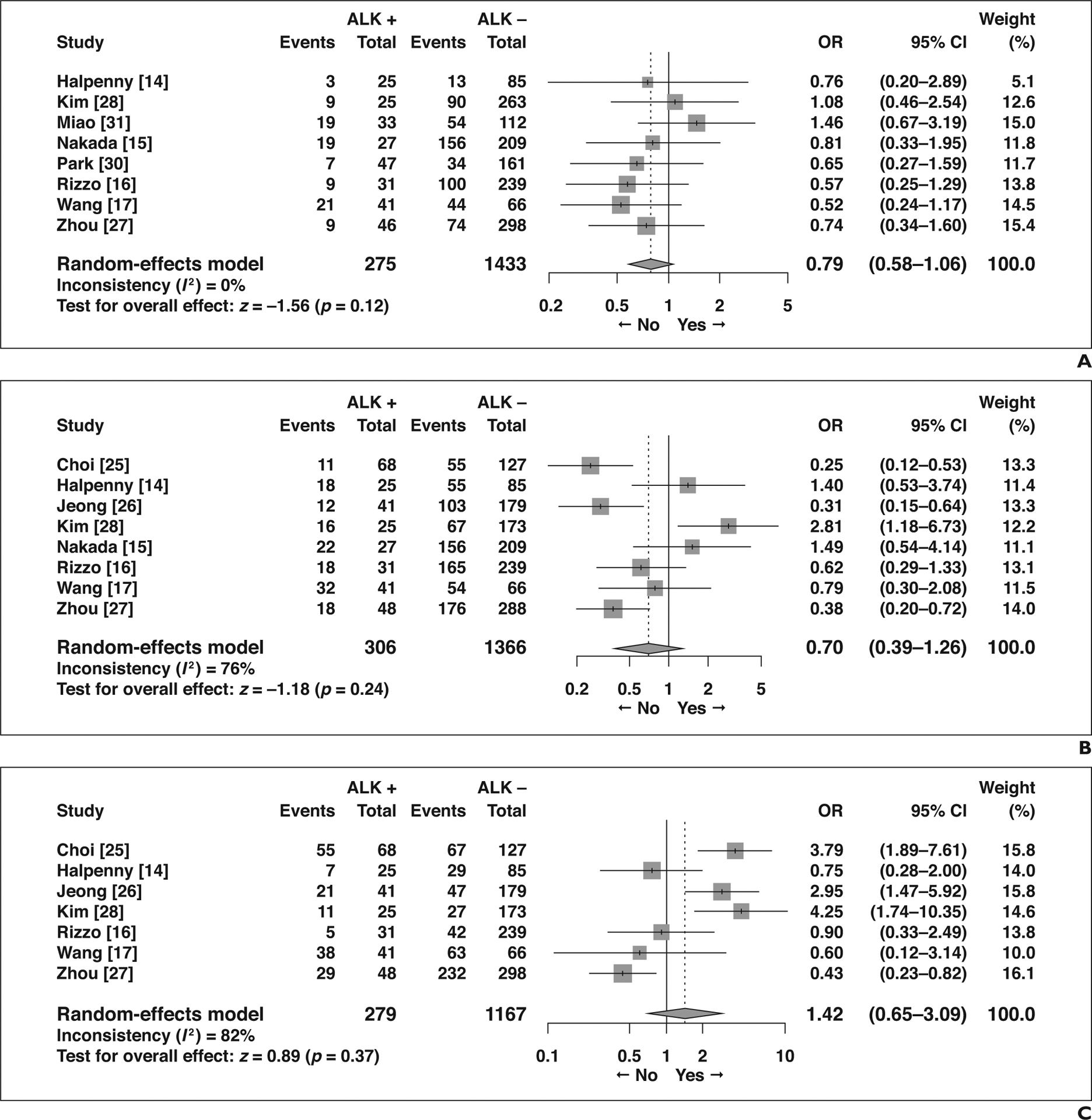
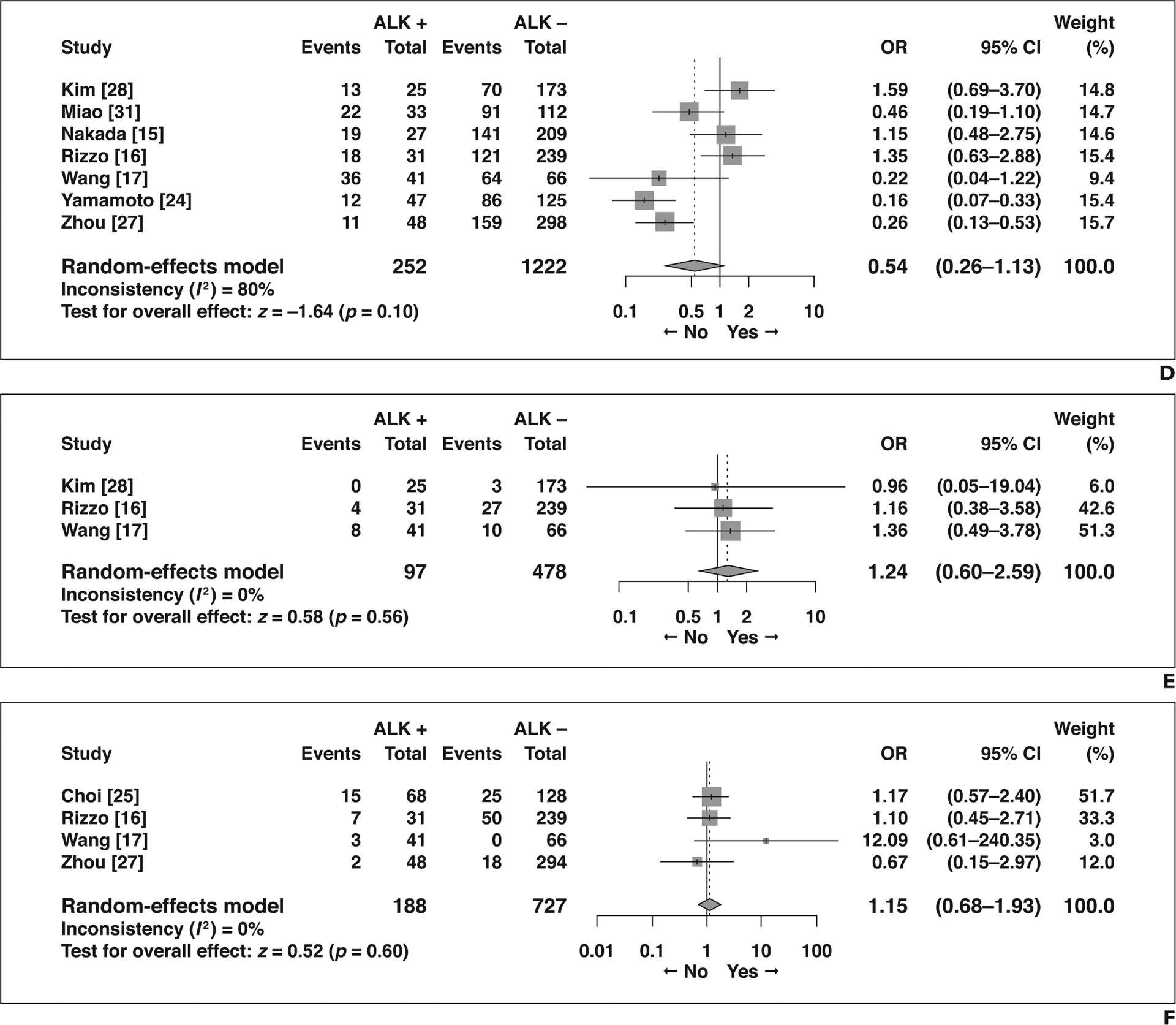
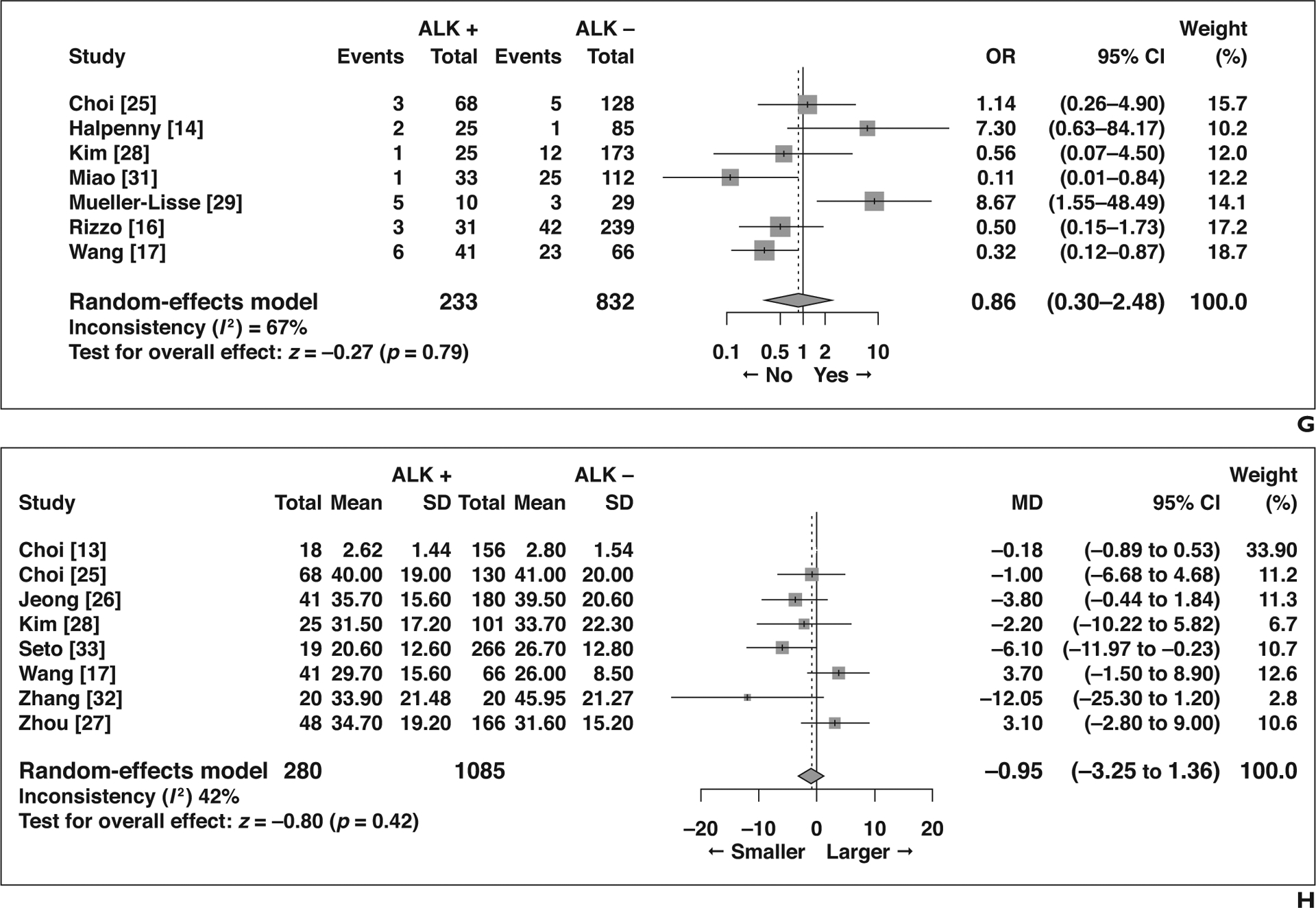
Forest plots of studies on association between CT features of primary tumor and anaplastic lymphoma kinase (ALK) rearrangement without statistical significance. Boxes indicate means, horizontal lines represent 95% CI, diamonds represent pooled indexes, and vertical dashed lines indicate pooled means. ALK + = with ALK rearrangement, ALK − = without ALK rearrangement.
A, Forest plot of odds ratio (OR) in studies assessing presence of air bronchogram.
B, Forest plot of OR in studies assessing presence of spiculated margin.
C, Forest plot of OR in studies assessing presence of lobulated margin.
D, Forest plot of odds ratio (OR) in studies assessing presence of pleural retraction.
E, Forest plot of OR in studies assessing presence of calcification.
F, Forest plot of OR in studies assessing presence of necrosis.
G, Forest plot of odds ratio (OR) in studies assessing presence of bubblelike lucency or cavitation.
H, Forest plot of mean difference (MD) in studies assessing size measured as continuous variable.
Features other than primary tumor and anaplastic lymphoma kinase rearrangement—
Five imaging features not related to the primary tumor showed significant association with ALK rearrangement (Fig. 5). Compared with patients without ALK rearrangement, patients with ALK rearrangement more frequently presented with N2 or N3 lymphadenopathy than with N0 or N1 lymphadenopathy (OR = 5.63 [95% CI, 2.99–10.61], p < 0.01), more frequently had lymphangitic carcinomatosis (OR = 3.46 [95% CI, 1.47–8.14], p < 0.01), and less frequently had lung metastasis (OR = 0.66 [95% CI, 0.47–0.93], p = 0.02). Pleural effusion was more common in NSCLC with ALK rearrangement (OR = 1.91 [95% CI, 1.24–2.95], p < 0.01), as was pleural metastasis (OR = 1.81 [95% CI, 1.23–2.67], p < 0.01). Although not statistically significant, bone metastasis tended to be less frequent in patients with NSCLC with ALK rearrangement than in those without (OR = 0.44 [95% CI, 0.17–1.11], p = 0.08).
Fig. 5—
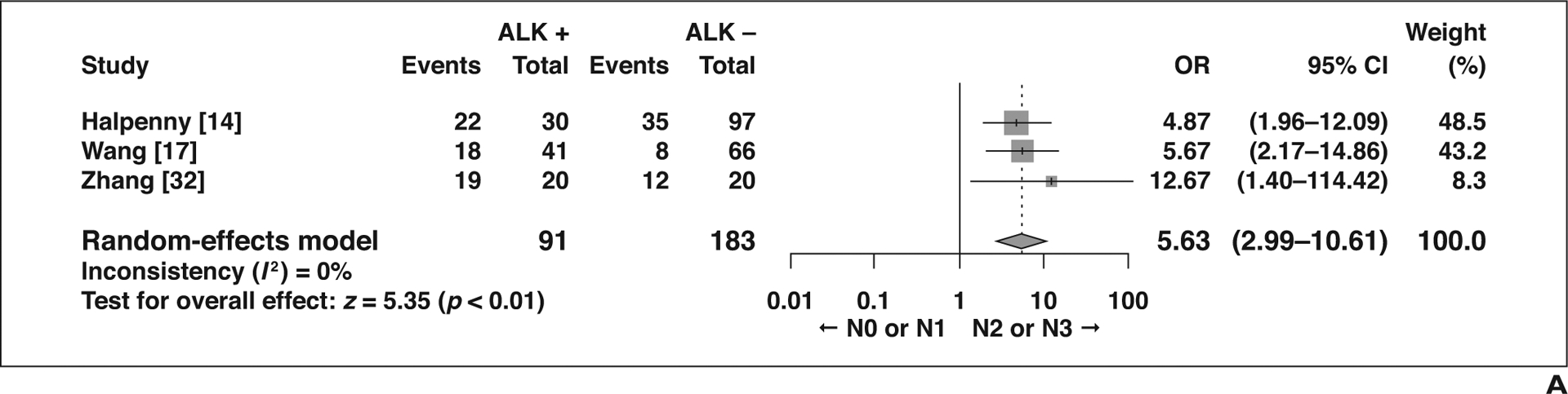
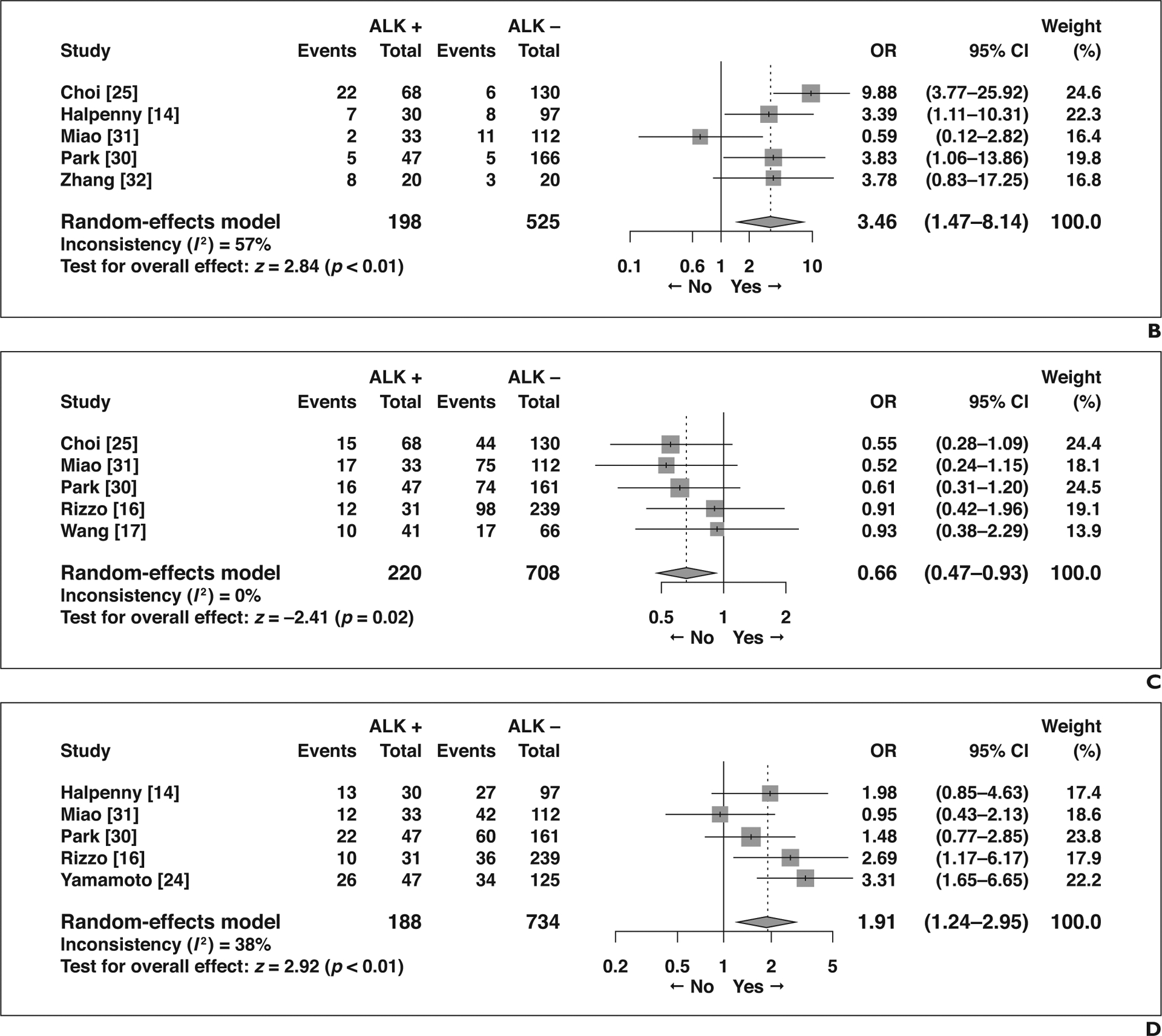
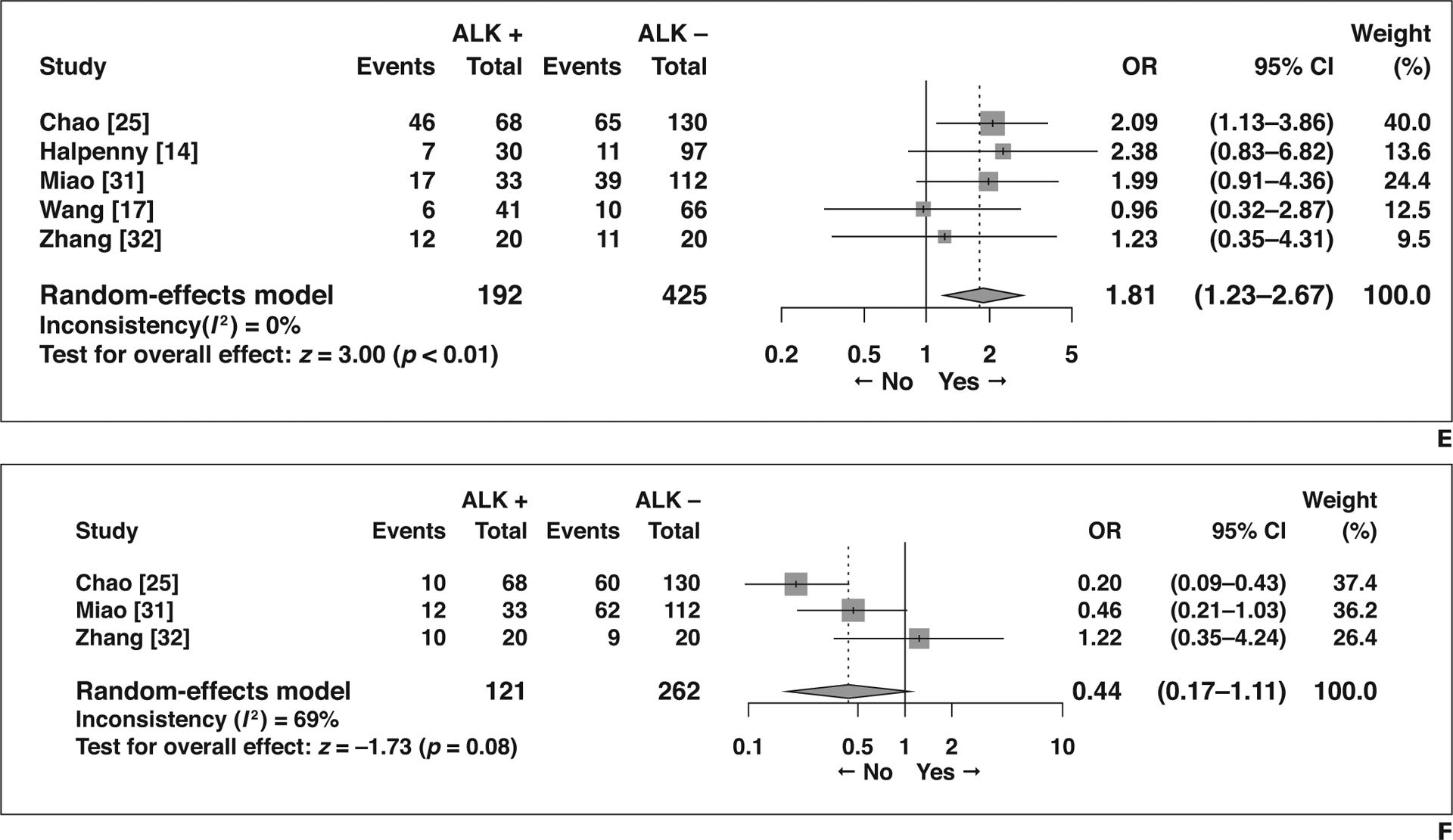
Forest plots of studies on association between CT features of tumors other than primary tumor and anaplastic lymphoma kinase (ALK) rearrangement. Boxes indicate means, horizontal lines represent 95% CI, diamonds represent pooled indexes, and vertical dashed lines indicate pooled means. ALK + = with ALK rearrangement, ALK − = without ALK rearrangement.
A, Forest plot of odds ratio (OR) in studies assessing N0 or N1 versus N2 or N3 lymphadenopathy.
B, Forest plot of odds ratio (OR) in studies assessing presence of lymphangitic carcinomatosis.
C, Forest plot of OR in studies assessing presence of lung metastasis.
D, Forest plot of OR in studies assessing presence of pleural effusion.
E, Forest plot of odds ratio (OR) in studies assessing presence of pleural metastasis.
F, Forest plot of OR in studies assessing presence of bone metastasis.
Publication Bias
Publication bias could only be tested for density (n = 11), and significant publication bias was not suggested with either the funnel plot or the Egger test (p = 0.1504) (Fig. 6). Other CT features included fewer than 10 studies, so publication bias was not statistically evaluated.
Fig. 6—
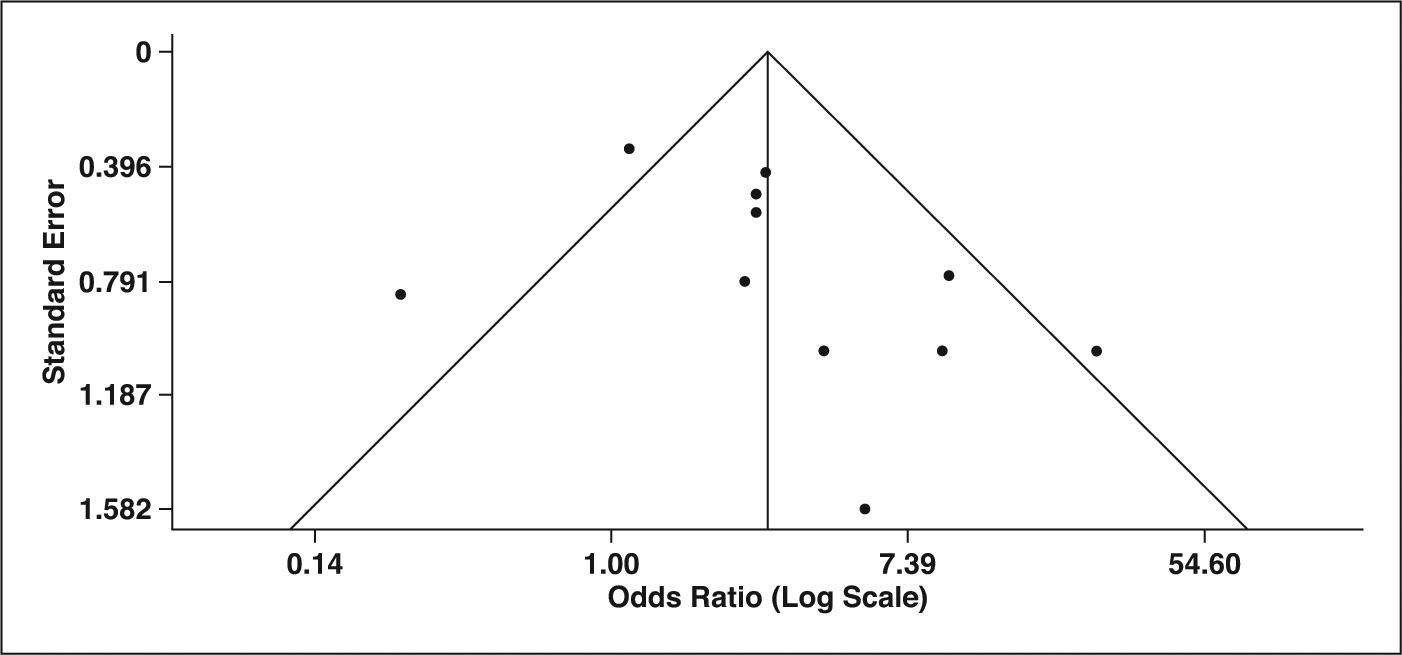
Funnel plot and Egger test for publication bias evaluation of CT feature of density.
Heterogeneity
CT features with significant heterogeneity were as follows with corresponding I2 values: bubble lucency or cavitation (67%), lobulated margin (82%), spiculated margin (76%), bone metastasis (69%), pleural retraction (80%), density (60%), and lymphangitic carcinomatosis (57%). The other features did not show significant heterogeneity (0–44%).
Discussion
In this meta-analysis, we investigated the CT features of NSCLC with ALK rearrangement and identified multiple CT features associated with ALK rearrangement. Molecular testing including EGFR, ROS1, and ALK is recommended for patients with NSCLC with an adenocarcinoma component or nonsquamous cell type [8, 34]. However, this testing is not universally performed and may not be considered because of several factors including low prevalence, high cost, and technical limitations [32]. In addition, performing a biopsy whenever an ALK rearrangement is suspected during treatment may not be feasible. Furthermore, even though several clinical and demographic characteristics of ALK rearrangement have been reported, many, such as female predominance and a history of never or light smoking, overlap with EGFR mutation, a more common genetic alteration, with only few differential points (e.g., ALK rearrangement and EGFR mutation are more likely to be found in younger and old patients, respectively), limiting the ability to accurately predict the presence of ALK rearrangement with clinical and demographic information alone [14, 17, 35]. Therefore, specific CT features of NSCLC with ALK rearrangement would help radiologists to better understand the imaging phenotype of this tumor subtype and thus raise clinical suspicion of possible ALK rearrangement during the process of image interpretation. In the appropriate clinical setting, this information could in turn prompt molecular testing and early initiation of treatment with ALK inhibitors.
With regard to the CT features of the primary tumor, we found that solid density, central location, size 3 cm or smaller, and low attenuation on contrast-enhanced CT were significantly associated with the presence of ALK rearrangement. Solid morphology and low attenuation on contrast-enhanced CT may reflect a solid signet-ring cell pattern and abundant intra- or extracytoplasmic mucin (or both), which has been reported in studies of histopathologic analysis of ALK-rearranged lung cancers [36, 37]. NSCLC with ALK rearrangement appeared as solid lesions in most of the included studies, unlike NSCLC with EGFR mutation, which is known to manifest as part-solid lesions [38]. In fact, among nine studies reporting an association between density and ALK status, only one [29] observed less-solid components in patients with ALK rearrangement compared with those without ALK rearrangement. However, that study analyzed CT images when disease progressed or recurred after at least one line of treatment, such as chemotherapy or radiotherapy. Although we speculate that posttreatment status may have influenced imaging of the primary tumor, further studies investigating imaging findings of treated ALK-rearranged tumors are warranted to elucidate this issue. In the meta-analysis, NSCLC with ALK rearrangement tended to frequently manifest as a nodule (size ≤ 3 cm) compared with NSCLC without ALK rearrangement, which frequently manifested as a mass (> 3 cm). Similarly, Park et al. [39] observed that even in the advanced stage, NSCLC with ALK rearrangement tended to manifest as a nodule (≤ 3 cm). However, caution is warranted because the difference in diameter may result from factors other than genetic mutation, particularly delay from disease onset.
Regarding CT features other than the primary tumor, ALK-rearranged NSCLC more frequently showed N2 or N3 lymphadenopathy, lymphangitic carcinomatosis, pleural effusion, and pleural metastasis. These findings possibly reflect a pathophysiologic tendency for lymphangitic rather than hematogenous spread [40, 41]. This pattern corroborates previous studies reporting higher frequency of lymph node involvement and lymphatic spread in NSCLC with ALK rearrangement, which was confirmed on pathology in surgically resected specimens [33, 42, 43]. Taking into consideration this affinity for extensive lymphatic spread despite the tendency for smaller primary tumor size, special care should be taken to find such features during routine interpretation of CT in patients with ALK-rearranged NSCLC.
Contrary to the finding of frequent lymphatic spread, we found that lung metastasis was significantly less common in ALK-rearranged NSCLC. Nevertheless, during the process of pooling data for lung metastasis, various heterogeneous descriptors were subsumed into the category of lung metastasis. Specifically, two studies [16, 17] reported either satellite nodules in the same lobe or nodules in different lobes, two others [30, 31] evaluated “lung metastasis,” and another [25] specifically categorized the pattern of lung metastasis into miliary and scattered metastasis. Unfortunately, meta-analytically pooling the results for each specific type of lung metastasis was not feasible because of the paucity of studies assessing them, so we placed them into a broader category of “lung metastasis.” Caution is warranted considering the heterogeneity in the exact definitions of “lung metastasis” used in these studies, and future studies evaluating each specific finding will help us understand the patterns of lung metastasis of ALK-rearranged lung cancers.
Some CT features showed a trend for differences between NSCLC with and without ALK rearrangement, albeit without statistical significance. Among them, air bronchogram and pleural retraction, which tended to be less frequent in NSCLC with ALK rearrangement, are well-recognized imaging features favoring NSCLC with EGFR mutation [44, 45]. Firm conclusions cannot be reached because the group without ALK rearrangement included EGFR-mutated cancers in some studies but in others also included those with K-ras mutations and wild type. Considering that ALK rearrangements and EGFR mutations are mutually exclusive, we speculate that further studies with larger numbers of patients may provide a clearer answer whether these CT features can be used to differentiate between ALK-rearranged and EGFR-mutated NSCLC [46]. In addition, regarding tumor margin (spiculated or lobulated) and bone metastasis, although an overall trend was recognized, we found conflicting results between included studies with a large amount of heterogeneity, mandating well-designed studies for further clarification.
This meta-analysis had some limitations. First, many imaging descriptors were unable to be pooled meta-analytically because of either a small number of studies or a lack of detailed definitions. Low prevalence of ALK rearrangement in NSCLC inevitably limits the total number of patients in each study that could be included in the meta-analysis, resulting in low statistical power and a few conflicting results between some studies. Nevertheless, through meticulous systematic searching and meta-analysis, we were able to include a relatively large number of 528 patients with ALK rearrangement and to find a number of statistically significant imaging features. Considering that this is the first meta-analysis to our knowledge to summarize CT features of NSCLC with ALK rearrangement with a large number of patients, it may contribute to future studies by providing more comprehensive understanding of the radiogenomics of ALK rearrangement. Second, even though the reference standard for the molecular diagnosis for ALK rearrangement is FISH, the detection methods used in included studies varied, including not only FISH but also IHC and PCR. However, according to the updated guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology regarding molecular testing in patients with lung cancer [34], IHC is an equivalent alternative to FISH for ALK testing, and PCR may be able to detect common fusions involving ALK. Third, half of the included studies performed biopsy for molecular testing for ALK rearrangement, raising concern for increased risk for sampling error. However, according to Kim et al. [47], in the case of determination of oncogenic drivers such as ALK in patients with lung cancer, mutational profiles of driver genes were the same in both biopsy and surgical samples, possibly because these oncogenic driver genes are widespread throughout the tumor, suggesting the possibility of sampling error to be low. Fourth, all included studies (n = 16) were retrospective. Prospective studies may be needed to confirm our results. Fifth, when results from readers with different levels of experience were reported, we used those of the most experienced reader. However, the interobserver agreement for CT features for differentiating cancers with and without ALK rearrangement was substantial or almost perfect (κ = 0.68–0.97) [14, 16, 24]. This level of agreement is a prerequisite for these CT features to be used for predicting ALK rearrangement. Last, multiple CT features had considerable heterogeneity. Lack of meta-regression or sensitivity analysis because of a limited number of included studies in each imaging category may hinder generalization of our results.
Conclusion
In conclusion, our meta-analysis found that NSCLC with ALK rearrangement has CT features distinctive from that without ALK rearrangement. These imaging biomarkers may help identify patients likely to have ALK rearrangement before molecular testing and raise clinical suspicion for this molecular subtype, thereby initiating prompt molecular testing and subsequent proper personalized treatment such as ALK inhibitors.
Acknowledgments
This study was funded in part by the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center.
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics In: Ahmad A, Gadgeel S, eds. Lung cancer and personalized medicine. Cham, Switzerland: Springer International Publishing, 2015:1–19 [Google Scholar]
- 2.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448:561–566 [DOI] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368:2385–2394 [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, et al. ; ALEX Trial Investigators. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377:829–838 [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018; 379:2027–2039 [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018; 19:1654–1667 [DOI] [PubMed] [Google Scholar]
- 8.Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non-small cell lung cancer, version 5.2018. J Natl Compr Canc Netw 2018; 16:807–821 [DOI] [PubMed] [Google Scholar]
- 9.Ulrich SH. NCCN trends highlights: targeted therapy in non-small cell lung cancer. National Comprehensive Cancer Network website. www.nccn.org/about/news/ebulletin/ebulletindetail.aspx?ebulletinid=449. Accessed January 2, 2019 [Google Scholar]
- 10.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012; 366:883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jamal-Hanjani M, Wilson GA, McGranahan N, et al. ; TRACERx Consortium. Tracking the evolution of non-small-cell lung cancer. N Engl J Med 2017; 376:2109–2121 [DOI] [PubMed] [Google Scholar]
- 12.Rutman AM, Kuo MD. Radiogenomics: creating a link between molecular diagnostics and diagnostic imaging. Eur J Radiol 2009; 70:232–241 [DOI] [PubMed] [Google Scholar]
- 13.Choi H, Paeng JC, Kim DW, et al. Metabolic and metastatic characteristics of ALK-rearranged lung adenocarcinoma on FDG PET/CT. Lung Cancer 2013; 79:242–247 [DOI] [PubMed] [Google Scholar]
- 14.Halpenny DF, Riely GJ, Hayes S, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer 2014; 86:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakada T, Okumura S, Kuroda H, et al. Imaging characteristics in ALK fusion-positive lung adenocarcinomas by using HRCT. Ann Thorac Cardiovasc Surg 2015; 21:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizzo S, Petrella F, Buscarino V, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol 2016; 26:32–42 [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Schabath MB, Liu Y, et al. Clinical and CT characteristics of surgically resected lung adenocarcinomas harboring ALK rearrangements or EGFR mutations. Eur J Radiol 2016; 85:1934–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151:264–269, W64 [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, et al. ; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536 [DOI] [PubMed] [Google Scholar]
- 20.Jackson D, White IR, Riley RD. Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 2012; 31:3805–3820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Green S, eds. Recommendations on testing for funnel plot asymmetry Cochrane website. handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm. Updated March 2011. Accessed December 31, 2018 [Google Scholar]
- 23.Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012; 77:319–325 [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S, Korn RL, Oklu R, et al. ALK molecular phenotype in non-small cell lung cancer: CT radiogenomic characterization. Radiology 2014; 272:568–576 [DOI] [PubMed] [Google Scholar]
- 25.Choi CM, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015; 275:272–279 [DOI] [PubMed] [Google Scholar]
- 26.Jeong CJ, Lee HY, Han J, et al. Role of imaging biomarkers in predicting anaplastic lymphoma kinase-positive lung adenocarcinoma. Clin Nucl Med 2015; 40:e34–e39 [DOI] [PubMed] [Google Scholar]
- 27.Zhou JY, Zheng J, Yu ZF, et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 2015; 25:1257–1266 [DOI] [PubMed] [Google Scholar]
- 28.Kim TJ, Lee CT, Jheon SH, Park JS, Chung JH. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann Thorac Surg 2016; 101:473–480 [DOI] [PubMed] [Google Scholar]
- 29.Mueller-Lisse UG, Zimmermann HA, Reiners C, et al. Distinct computed tomography features of non-small-cell lung cancer in a European population with EML4-ALK translocation. Memo 2016; 10:94–102 [Google Scholar]
- 30.Park J, Kobayashi Y, Urayama KY, Yamaura H, Yatabe Y, Hida T. Imaging characteristics of driver mutations in EGFR, KRAS, and ALK among treatment-naïve patients with advanced lung adenocarcinoma. PLoS One 2016; 11:e0161081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miao Y, Zhu S, Li H, et al. Comparison of clinical and radiological characteristics between anaplastic lymphoma kinase rearrangement and epidermal growth factor receptor mutation in treatment naïve advanced lung adenocarcinoma. J Thorac Dis 2017; 9:3927–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang LWN, Li M, Ying J, Ouyang H, Yang C, Lv L. The diagnostic and follow-up role of radiological examination in advanced lung adenocarcinoma with anaplastic lymphoma kinase gene rearrangement. Int J Clin Exp Med 2017; 10:12782–12789 [Google Scholar]
- 33.Seto K, Kuroda H, Yoshida T, et al. Higher frequency of occult lymph node metastasis in clinical N0 pulmonary adenocarcinoma with ALK rearrangement. Cancer Manag Res 2018; 10:2117–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Mol Diagn 2018; 20:129–159 [DOI] [PubMed] [Google Scholar]
- 35.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009; 27:4247–4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida A, Tsuta K, Nakamura H, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 2011; 35:1226–1234 [DOI] [PubMed] [Google Scholar]
- 37.Jokoji R, Yamasaki T, Minami S, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J Clin Pathol 2010; 63:1066–1070 [DOI] [PubMed] [Google Scholar]
- 38.Cheng Z, Shan F, Yang Y, Shi Y, Zhang Z. CT characteristics of non-small cell lung cancer with epidermal growth factor receptor mutation: a systematic review and meta-analysis. BMC Med Imaging 2017; 17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J, Yamaura H, Yatabe Y, et al. Anaplastic lymphoma kinase gene rearrangements in patients with advanced-stage non-small-cell lung cancer: CT characteristics and response to chemotherapy. Cancer Med 2014; 3:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bilgi Z, Colson YL. Lymphatic drainage of the pleura and its effect on tumor metastasis and spread. Turkish Thoracic Society website. www.toraks.org.tr/Download.aspx?book=609. Published September 2009. Accessed June 9, 2019 [Google Scholar]
- 41.Broaddus VC, Wiener-Kronish JP, Berthiaume Y, Staub NC. Removal of pleural liquid and protein by lymphatics in awake sheep. J Appl Physiol (1985) 1988; 64:384–390 [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Lei J, Wang QZ, Li J, Wu L. Clinical characteristics of patients with non-small cell lung cancers harboring anaplastic lymphoma kinase rearrangements and primary lung adenocarcinoma harboring epidermal growth factor receptor mutations. Genet Mol Res 2015; 14:12973–12983 [DOI] [PubMed] [Google Scholar]
- 43.Takamochi K, Takeuchi K, Hayashi T, Oh S, Suzuki K. A rational diagnostic algorithm for the identification of ALK rearrangement in lung cancer: a comprehensive study of surgically treated Japanese patients. PLoS One 2013; 8:e69794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Shi J, Soodeen-Lalloo AK, et al. Air bronchogram: a potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer 2016; 98:22–28 [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Kim J, Qu F, et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology 2016; 280:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013; 19:4273–4281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HK, Lee HY, Choi YL, et al. Assessment of intratumoral heterogeneity of oncogenic driver mutations in surgically-resected lung adenocarcinoma: implications of percutaneous biopsy-based molecular assay for target-directed therapy. Anticancer Res 2014; 34:707–714 [PubMed] [Google Scholar]


