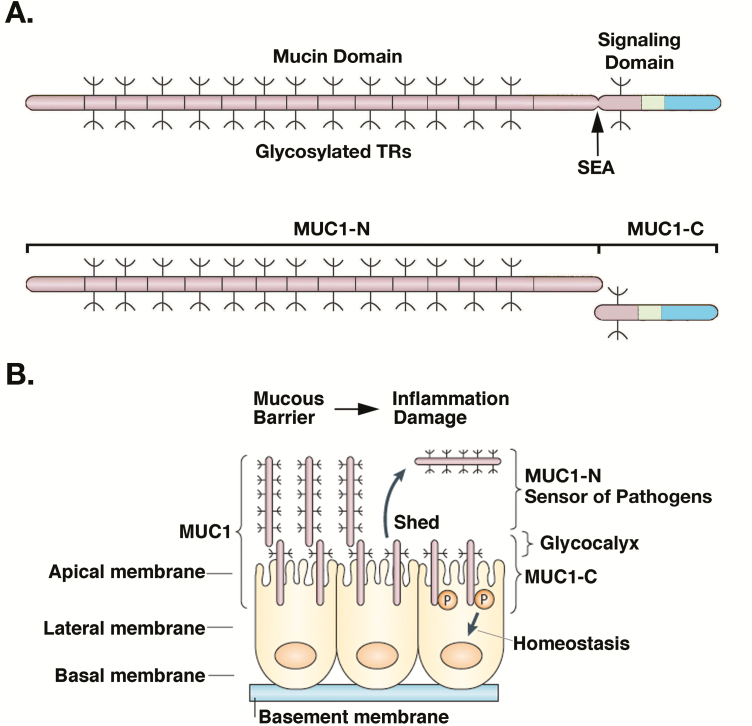
Figure 1.
The MUC1 protein is cleaved into MUC1-N and MUC1-C subunits that form a complex at the epithelial cell apical membrane to respond to the microbiome and loss of homeostasis. (A) MUC1 is translated as a single polypeptide that includes (i) a characteristic mucin-like domain of glycosylated proline, threonine and serine (PTS) rich TRs and (ii) a signaling domain that evolved in mammals as an adaptation to environmental stress. MUC1 undergoes auto-cleavage at a SEA domain, resulting in MUC1 N-terminal (MUC1-N) and C-terminal (MUC1-C) subunits that, in turn, form a non-covalent heterodimer (8). The MUC1-N and MUC1-C nomenclature defines positioning of the subunits after cleavage and distinguishes them from genetic isoforms designated by Greek characters, such as ERα and ERβ, among others. Figure modified from Kufe (15). (B) MUC1-N extends as a rod-like structure into and beyond the glycocalyx as a component of the protective mucous barrier. MUC1-N is tethered to the cell membrane in a complex with the transmembrane MUC1-C subunit. Mechanical disruption of the complex in the response to loss of homeostasis results in shedding of MUC1-N into the mucous barrier and activation of MUC1-C for the intracellular transduction of signals to reestablish homeostasis. Figure modified from Kufe (6).