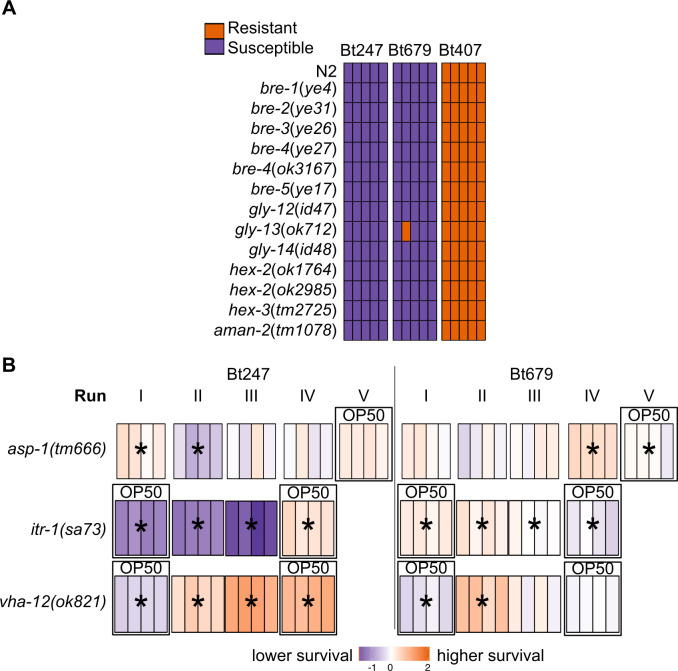
Fig 6. Specific responses to Bt247 and Bt679 do not involve the bre genes or the necrosis pathway.
(A) A binary score considered resistance of glycosylation-deficient mutants to pathogen exposure as >70% survival of ~300 worms on plate. N = 5. Each bar represents the score of a single plate. Our results show that glycosylation-deficient mutants are susceptible to Bt679 and Bt247. (B) Difference in survival between the N2 control and the knockout mutants of the: terminal necrosis protein aspartase asp-1(tm666), inositol triphosphate receptor ion channel itr-1(sa73), and vacuolar proton translocating ATPase vha-12(ok821). Asterisks show significant differences between mutant and the wildtype N2 control. Infection of Bt247 led to inconsistent survival phenotypes of the asp-1(tm666) mutant animals, including significantly higher survival, significantly lower survival, and no survival difference in separate runs of the experiment. Similar levels of variation were also found upon Bt247 infection of asp-1(tm666) mutant animals, in which elt-2 was inactivated by RNAi. The necrosis-deficient mutant of another pathway component, itr-1(sa73), had significantly higher survival rate on Bt679 and lower survival rate on Bt247 compared to control worms. The necrosis-deficient mutant of yet an additional pathway component, vha-12(ok821), had higher survival rate on Bt247 compared to control worms and had inconsistent survival phenotypes on Bt679. Since knockout mutants of different necrosis pathway components show different survival phenotypes upon infection, we conclude that deficiency in necrosis is unlikely to have a strong effect on tolerance to Bt247. The data represented in the heatmap and statistics as in Fig 3E. In (B) worms were grown on RNAi E. coli HT115 plates or on NGM plates seeded with E. coli OP50 (indicated by ‘OP50’ above a run).