FIG 11.
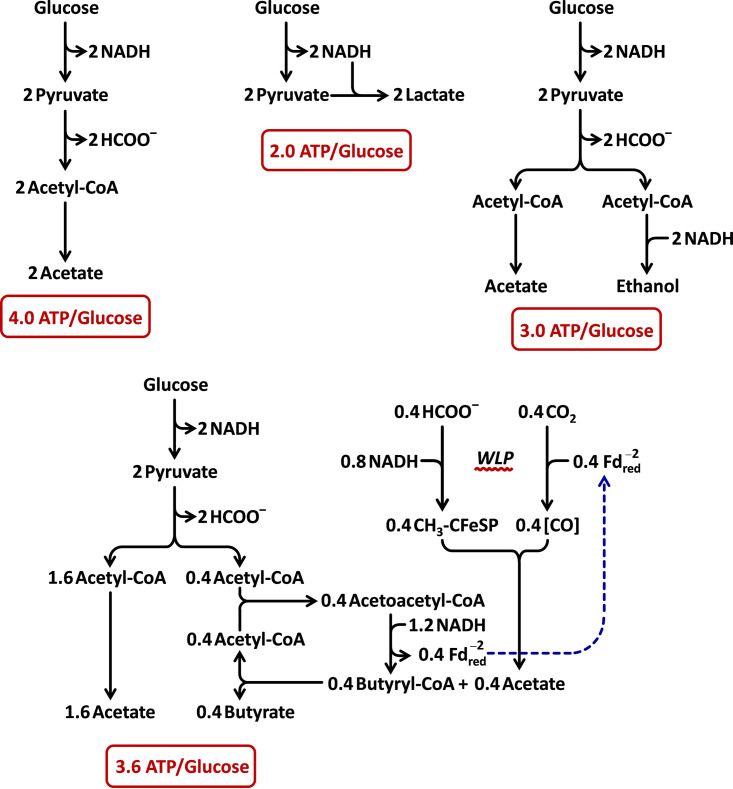
Approximate ATP yields from glucose in various anaerobic fermentation pathways and using the Wood-Ljungdahl pathway coupled to butyrate formation. The efficiencies of ATP production from glucose are compared. For simplicity, contributions from chemiosmotic mechanisms and membrane complexes, such as Rnf, are excluded. In general, the efficiency of ATP production is highest when organic products of the pathway are not required to serve as electron acceptors, which increases the amount of acetyl-CoA available to produce ATP via SLP. A maximal level of 4.0 ATPs/glucose is expected, provided that another pathway is used to fully regenerate NAD+ and assuming that pyruvate is converted to acetyl-CoA without producing additional redox equivalents either as NADH or reduced ferredoxin. This assumption is reasonable for C. difficile given that formate is produced even in the absence of added H2 and that glycine and alanine are consumed in a 1:1 stoichiometry in the Gly/Ala fermentation (see Results and Fig. 4). By comparison, the lactic acid fermentation yields the lowest ATP/glucose ratio of 2.0, because no SLP from acetyl-CoA is possible. Diversion of one-half of the acetyl-CoA to ethanol regenerates the necessary amount of NAD+ and improves the ratio to 3.0. Connection of the WLP pathway to the pathway for butyrate formation from acetyl-CoA results in a further increase to 3.6, closer to the theoretical maximum. A total of 5 NADHs are consumed per mol butyrate produced by this arrangement: 2 NADHs are used by the WLP to produce each acetate, which enters into the butyryl-CoA:acetate CoA transferase reaction to form butyrate and regenerate acetyl-CoA, and 3 NADHs are taken up for each acetoacetyl-CoA converted to butyryl-CoA (1 NADH from the 3-hydroxybutyryl-CoA dehydrogenase reaction, and 2 NADHs from the reduction of crotonyl-CoA involving electron bifurcation by the Bcd-EtfAB complex that also conserves energy by producing 1 , which is then used to reduce CO2 to CO in the WLP). However, only 2 NAD+s instead of 5 are needed per glucose oxidized so that formation of 0.4 mol butyrate is sufficient to balance the fermentation using butyrate/WLP coupling.