Figure 4.
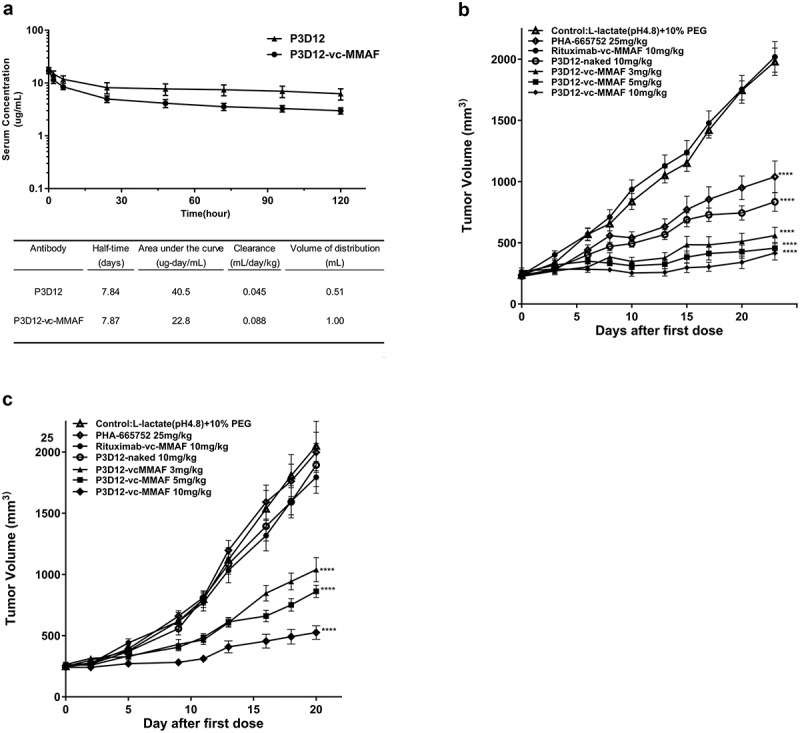
In vivo assessment of lead P3D12 cMet ADC.
(a) Time concentration curves of P3D12 and P3D12-vc-MMAF and their pharmacokinetic parameters in mice. C57BL/6 mice were injected via the vein with 1 mg/kg P3D12 and P3D12-vcMMAF. Plasma samples were analyzed by ELISA to determine the antibody or antibody-drug conjugate concentration. Pharmacokinetic parameters of P3D12 and P3D12-vc-MMAF antibody-drug conjugate were calculated with WinNonlin software. (b) Antitumor activity of P3D12 and P3D12-vc-MMAF in the high c-Met gastric cancer xenograft model MKN-45. P3D12 unconjugated antibody was administered intravenously at 10.0 mg/kg and P3D12-vcMMAF at 3.0, 5.0, and 10.0 mg/kg and compared to the control ADC Rituximab-vc-MMAF (Rituxan®: Rituximab, 10.0 mg/kg). Group size n = 9, Tumor volume was plotted as mean ± S.E.M. Statistical significance was determined with a two-way ANOVA followed by Bonferroni post hoc test (**** p < .0001 compared to control). (c) Antitumor activity of P3D12 and P3D12-vc-MMAF in low-medium c-Met lung cancer xenograft model H1975 with L858 R/T790 M-EGFR mutations. P3D12 unconjugated antibody was administered intravenously at 10.0 mg/kg and P3D12-vc-MMAF was at 3.0, 5.0, and 10.0 mg/kg and compared with the control ADC Rituximab-vc-MMAF (Rituxan®: Rituximab, 10.0 mg/kg). Group size n = 9, Tumor volume was plotted as mean ± S.E.M. 9 mice per group were used for the H1975 studies. Statistical significance was determined with a two-way ANOVA followed by Bonferroni post hoc test (**** p < .0001 compared to control).
