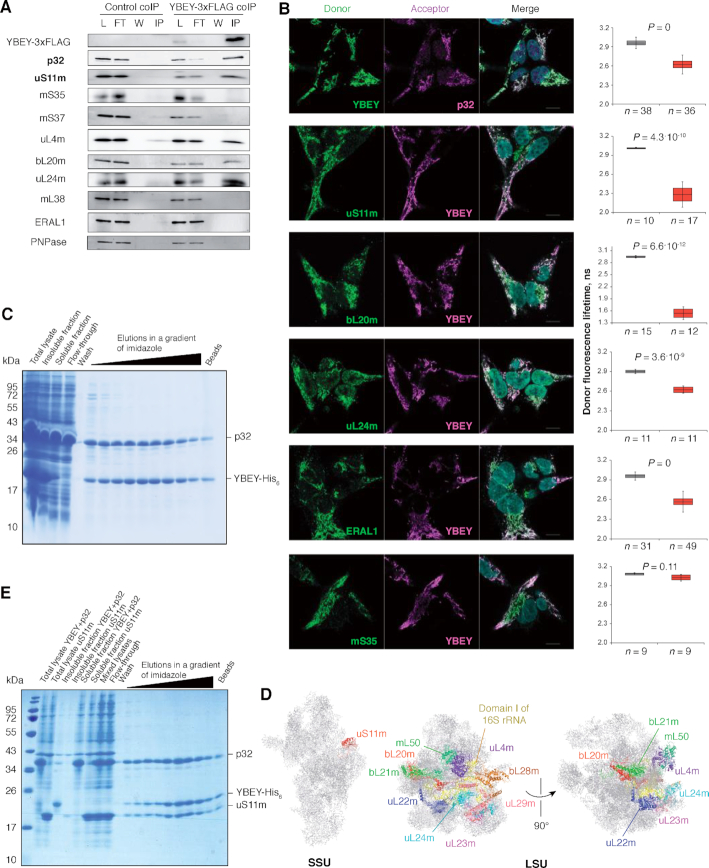
Figure 5.
YBEY interacts with p32 and a distinct set of mitoribosomal proteins. (A) Immunoprecipitation of YBEY-3 × FLAG from mitochondrial lysates copurifies p32 and select mitoribosomal proteins. Western blot analysis of lysate (L), flow-through (FT), wash (W) and immunoprecipitate (IP) fractions for WT control (not expressing FLAG-tagged baits) and stable YBEY-3 × FLAG-expressing HEK293T-REx cells is shown. The particularly highly enriched p32 and uS11m proteins are set in bold. See also Supplementary Table S6. (B) Indirect FLIM-FRET analysis of in situ interactions between YBEY and select mitochondrial proteins in HEK293T-REx cells. Representative images of staining of the corresponding partners with fluorescent antibodies forming a FRET couple are shown along with donor fluorescence lifetime measurements when the donor alone (grey boxes) or together with the acceptor (red boxes) is labelled. The boxes show the means and the 95% CI, the bars are SD. A significant decrease in the donor fluorescence lifetime in the presence of the acceptor (Bonferroni-corrected two-tailed Welch's test) is indicative of FRET between the two and, therefore, of a tight spatial proximity of the interactors. On all microscopy images, the donor is labelled in green and the acceptor in magenta. mS35 is an example of mitoribosomal protein that does not interact with YBEY. (C) Coexpression of human YBEY-His6 with tagless p32 in E. coli results in partial solubilization of the former and stoichiometric copurification of both on a Ni-agarose column. (D) Mapping of the mitoribosomal proteins copurifying with YBEY on the structure of the mammalian mitoribosome. Note the spatial clustering of the enriched LSU proteins around domain I of 16S rRNA. (E) A stable and stoichiometric ternary complex copurifies on Ni-agarose beads from mixed lysates of YBEY-His6/p32 and uS11m-expressing E. coli cells. See Supplementary Figure S9C for a control purification.