Fig. 5.
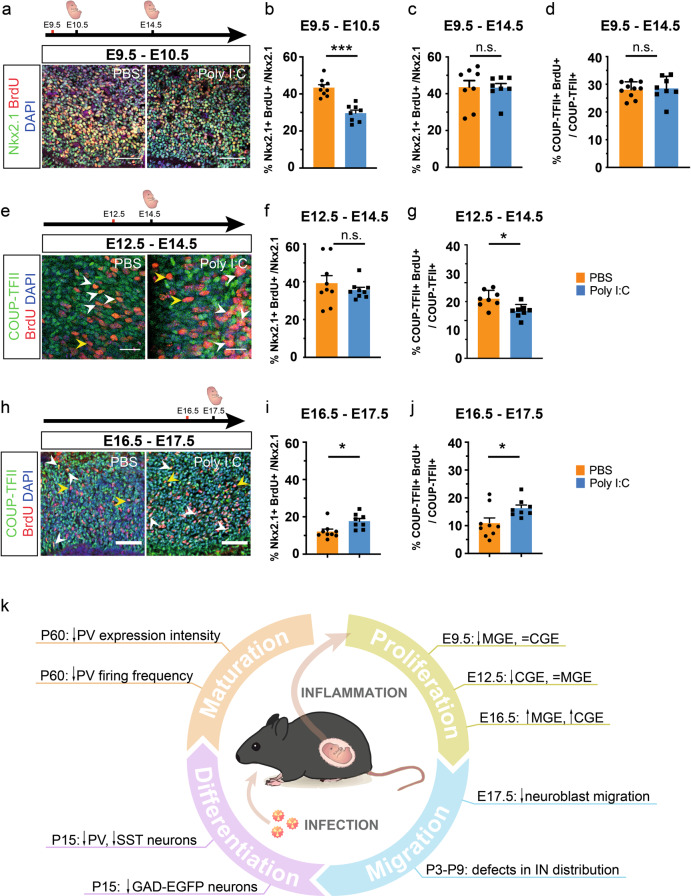
Acute and developmental stage-dependent impairment of progenitor proliferation due to maternal inflammation. a–c Maternal inflammation had acute effect on proliferation of MGE-derived Nkx2.1+ progenitors. Dams were injected with poly I:C at E9.5 and embryos assessed at E10.5 after a 2-h BrdU pulse-chase (a, b). Quantification of proliferating Nkx2.1+ BrdU+ cells showed that maternal inflammation resulted in reduction in Nkx2.1+ proliferating cells at E10.5 (b) (n = 9 and 8 for PBS and poly I:C, respectively) that did not sustain until E14.5 (c). COUP-TFII+ progenitors remained unaffected when studied at E14.5 (d). e–g Dams were injected with poly I:C at E12.5 and embryos assessed at E14.5 after a 2-h BrdU pulse-chase. Proliferation of CGE-derived COUP-TFII+ progenitors was reduced after maternal inflammation at E12.5 (e, g) (n = 9 and 8 for PBS and poly I:C, respectively) that correlated with the later neurogenic window of CGE-derived progenitors. In contrast, proliferation of Nkx2.1+ MGE-derived progenitors was unaffected at E14.5 (f). h–j Late maternal inflammation at E16.5 led to an increase in proliferation of both COUP-TFII+ (h, j) and Nkx2.1+ (i) progenitors (n = 8–9 for each PBS and Poly I:C). k Schematic representation of the findings in this manuscript. Maternal inflammation affects interneuron development at multiple stages such as proliferation, migration, differentiation and maturation leading to increased vulnerability to mental disorders. Central artwork was modified from [89]. Comparison of means by Student’s t-test in b–d, f, g, I, and j)(mean ± SEM are shown, *p < 0.05, **p < 0.005, ***p < 0.0005). Scale bars: 50 µm (a, e, h)