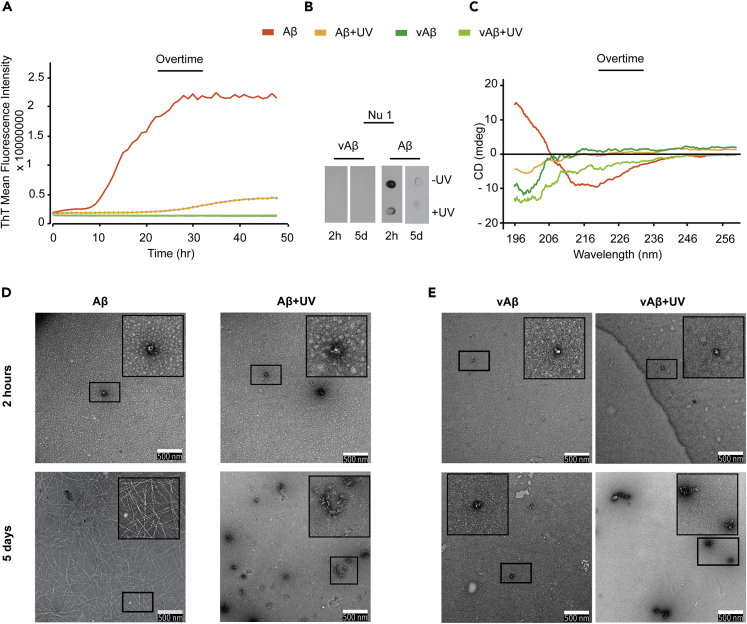
Figure 4.
UV-Induced DiY Cross-Linking of Early Aβ Assemblies Correlates with Formation of Stabilized Assemblies
(A) Th-T fluorescence spectrum shows the expected increase in fluorescence for assembling Aβ, but Aβ+UV Th-T fluorescence was significantly reduced. vAβ incubated in the absence or presence UV showed no Th-T fluorescence.
(B) Dot blotting using NU-1 antibody shows binding suggesting fewer oligomers in the oxidized Aβ+UV than in the unoxidized Aβ sample. No binding of NU-1 was observed for Aβ+UV 5 days post-UV exposure, but a small signal was detected in the Aβ-UV sample.
(C) CD at 5 days showed a high β-sheet content in the Aβ sample, whereas the oxidized Aβ+UV showed a loss of signal but indicated some random coil. Oxidized and unoxidized vAβ samples showed random coil conformation.
(D) TEM after 2 h and 5 days showed that the unoxidized Aβ at 2 h formed oligomers, which transformed into a network of fibers at 5 days. The oxidized Aβ+UV samples formed small assemblies at 2 h, some of which developed into amorphous-like assemblies at 5 days.
(E) vAβ does not assemble into amyloid fibrils, but vAβ+UV forms some amorphous aggregates after 5 days. A minimum of three independent experiments was repeated to ensure the reproducibility of the findings. Scale bars, 500 nm.