Figure 2.
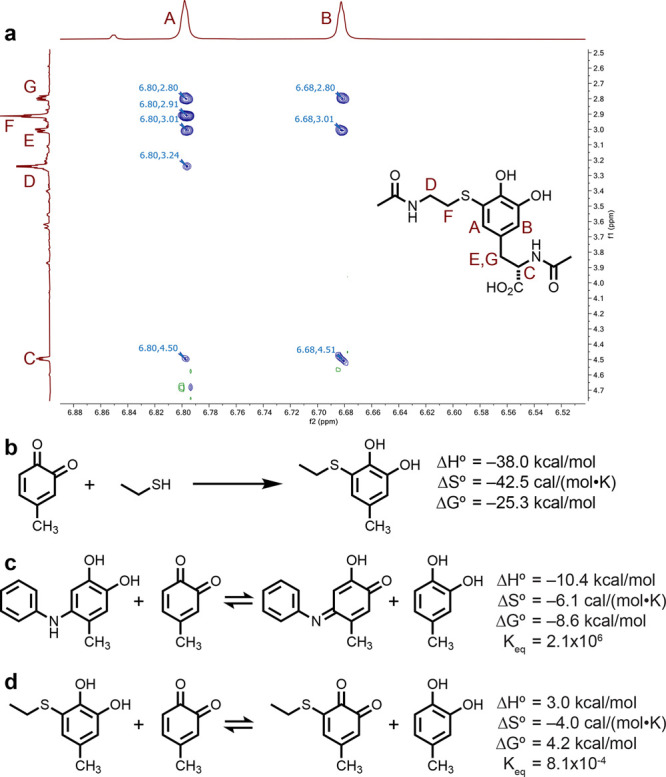
Structural analysis of thiol–o-quinone coupling products. (a) ROESY 1H NMR data (900 MHz) show a clear correlation between proton A and the corresponding signals from both the alkyl chain in N-acetylcysteamine (F) and the N-acetyltyrosine alkyl chain (E, G). (b) Energetics of the coupling reaction as estimated using DFT calculations. Geometry optimization and vibrational spectral calculations were conducted at the B3LYP-D3/6-31G** level, and final electronic energies were calculated at the ωB97M-V/6-311G++(3df,3pd) level. A CPCM solvation model was used in these calculations (see the Supporting Information for details and molecular coordinates). These data were combined to obtain the reported thermodynamic values. For comparison purposes, the reaction energies and equilibrium constants were estimated using this approach for the oxidation of the initial (c) aniline and (d) thiol coupling products with a methyl-substituted o-quinone.
