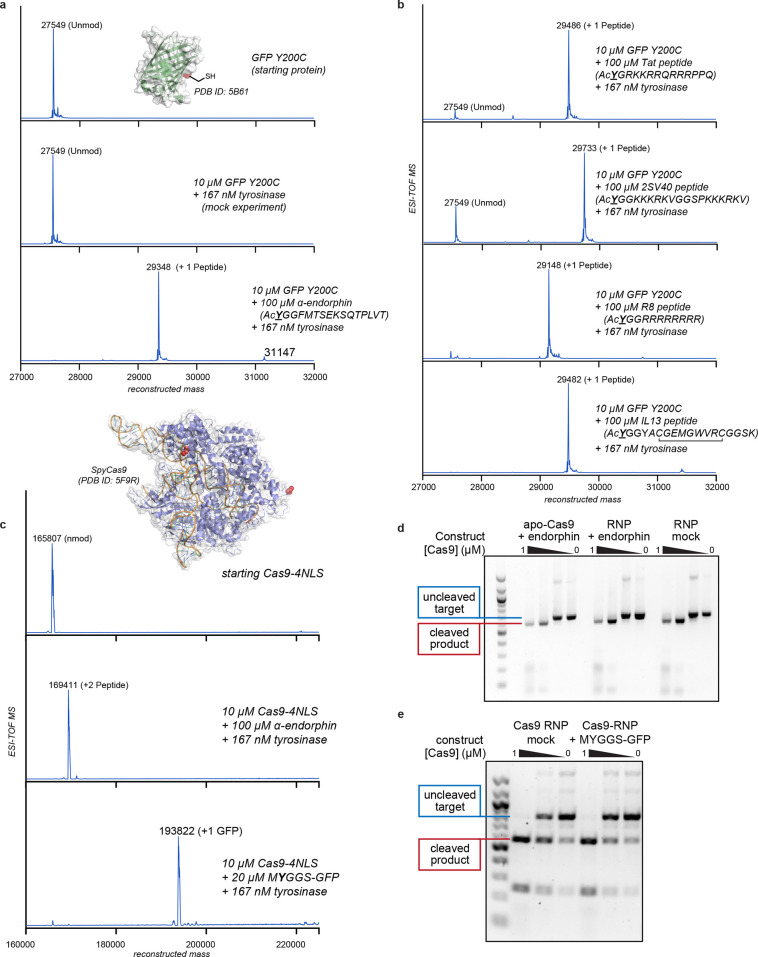
Figure 3.
Synthesis of protein–peptide and protein–protein conjugates using thiol-directed enzymatic oxidative coupling reactions. (a, b) A superfolder GFP (sfGFP) thiol mutant (Y200C) was used as a protein component to evaluate peptide coupling reactions. All of the reactions were run for 30 min at RT and characterized using ESI-TOF MS. (c–e) A CRISPR-Cas9 variant with two C-terminal and four N-terminal nuclear localization sequences (Cas9-4NLS) was modified with peptide and protein coupling partners. Cas9 has two surface thiols (Cys 80 and Cys 574, red in the attached structure) in the native sequence. (c) ESI-TOF MS data indicated complete coupling at both sites with the α-endorphin peptide. The coupling reaction was successful even when the coupling partner was sfGFP bearing an exposed tyrosine residue near the N-terminus (MYGGS-GFP). All Cas9 couplings were run on ice for 1 h. (d) The site-specific DNA cleaving ability of the Cas9–peptide conjugate was unchanged relative to an untreated control. This was true when the preassembled ribonucleoprotein (RNP) was modified directly or when the RNA-free protein (apo-Cas9) was used for the modification reaction. In the latter case, the single guide RNA strand was added before the DNA cleavage experiment. (e) The Cas9–GFP conjugate retained site-specific DNA cleaving ability despite the high degree of added steric bulk.