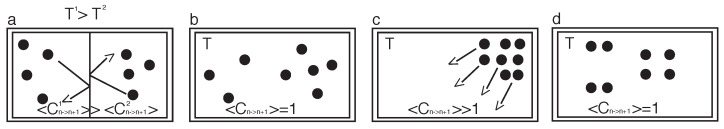
Figure 1.
Gedankenexperimente (a) An isolated system (indicated by double walls) is composed of two closed sub-systems with unequal temperature that can exchange heat through the single wall. The systems are filled with an ideal gas. The gas molecules indicated by spheres from the left sub-system (1) have a larger kinetic energy yielding a larger momentum change in average at the heat exchanging wall than the molecules from the right sub-system (2) when hitting the wall yielding a larger causality on the left sub-system when compared with the right sub-system (b) An ideal gas system in equilibrium. (c) A system in which the ideal gas molecules are concentrated on the right top corner. (d) The system is in thermodynamic equilibrium and a special state is selected.