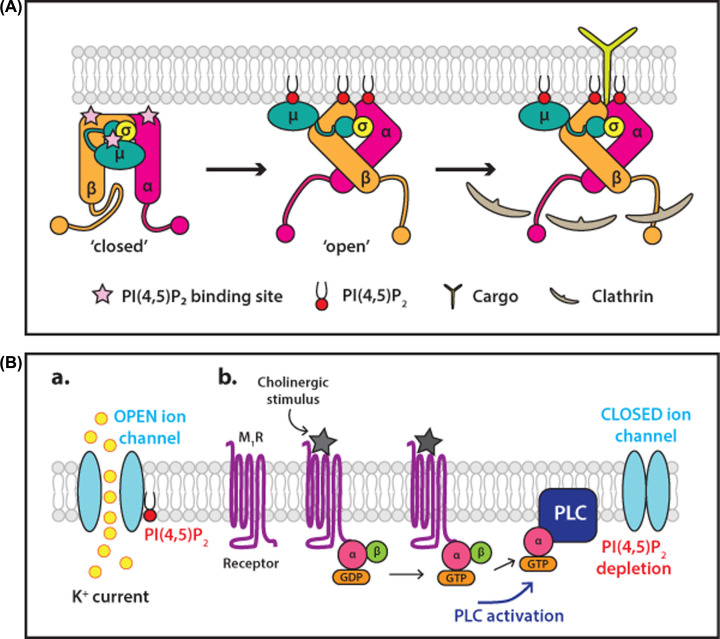
Figure 6. Examples of membrane peripheral and membrane integral proteins regulated by PI(4,5)P 2.
(A) Binding of PI(4,5)P2 to the protein complex, AP2, changes its conformation to allow cargo and clathrin interactions. The adaptor protein, AP2 is a complex of four proteins consisting of a core comprising the N-terminal domains of the α-and β2-adaptins in complex with the μ2 and σ2 subunits. The α, β2 and μ2 subunits all contain PI(4,5)P2 binding sites marked with pink stars. Long flexible linkers, referred to as hinge regions, connect the C-terminal appendage domains of α-and β2-adaptins to the core. AP2 exists in a closed conformation in the cytosol, in which the clathrin binding site is buried by interactions between the β2 hinge and the core and the cargo binding site on the μ2 subunit are also buried. Initially, the surface-exposed PI(4,5)P2 binding site on the α-and β2-adaptin interact with the lipid triggering an allosteric conformational change to an open conformation. This exposes the clathrin binding site on the β2 hinge as well as the PI(4,5)P2 and cargo binding sites of μ2. Figure adapted from [99]. (B) Regulation of potassium channels by PI(4,5)P2 depletion by PLC. Potassium channels are maintained in the open state when bound to PI(4,5)P2. Stimulation of the muscarinic M1 receptor by a cholinergic stimulus activates PLC to hydrolyse PI(4,5)P2. PI(4,5)P2 depletion results in closure of the ion channel. Abbreviations: M1R, M1 muscarinic receptor; PI(4,5)P2, phosphatidylinositol(4,5,)bisphosphate. Figure adapted from [154].